Advertisements
Advertisements
Question
How much heat energy is necessary to raise the temperature of 5 kg of water from 20°C to 100°C?
Solution
Given: Mass (m) = 5 kg, specific heat of water (c) = 1 kcal/kg °C
Change in temperature (ΔT) = 100 − 20 = 80°C
To find: Heat energy (Q)
Formula: Q = m c ΔT
Calculation:
According to the principle of heat exchange,
Energy supplied to water = Energy gained by water
From the formula,
Q = 5 × 1 × 80 = 400 kcal
Heat energy necessary to raise the temperature of the water is 400 kcal.
APPEARS IN
RELATED QUESTIONS
A geyser heats water flowing at the rate of 3.0 litres per minute from 27 °C to 77 °C. If the geyser operates on a gas burner, what is the rate of consumption of the fuel if its heat of combustion is 4.0 × 104 J/g?
Define heat capacity and state its SI unit.
Specific heat capacity of substance A is 3.8 J g-1K-1 whereas the specific heat capacity of substance B is 0.4 J g-1 K-1
(i) Which of the two is a good conductor of heat?
(ii) How is one led to the above conclusion?
(iii) If substances A and B are liquids then which one would be more useful in car radiators?
Give a mathematical relation between Heat Capacity and Specific Heat Capacity.
State S.I. unit of specific heat capacity.
How will rise in sea level affect population in coastal countries?
The global warming has resulted:
(a) the increase in yield of crops
(b) the decrease in sea levels
(c) the decrease in human deaths
(d) the increase in sea levels
Ice-cream at 0°C feels colder than water at 0°C. Give reason for this observation.
Discuss how high specific heat capacity of water helps in formation of land and sea breeze.
A substance is in the form of a solid at 0°C. The amount of heat added to this substance and the temperature of the substance are plotted on the following graph:

If the specific heat capacity of the solid substance is 500 J/kg °G, find from the graph, the mass of the substance.
Solve the following problem.
Specific latent heat of vaporization of water is 2.26 × 106 J/kg. Calculate the energy needed to change 5.0 g of water into steam at 100 ºC.
Solve the following problem.
What is the specific heat of metal if 50 cal of heat is needed to raise 6 kg of the metal from 20°C to 62 °C?
Read this activity and answer the following questions.
- Take three spheres of iron, copper and lead. the lead of equal mass.
- Put all the three spheres in boiling water in the beaker for some time.
- Take the three spheres out of the water.
- All the spheres will be at a temperature 100 °C.
- Put them immediately on the thick slab of wax.
- Note, the depth that each of the sphere goes into the wax.
Questions:
- Which property is determined from this activity?
- Give name to that property.
- Explain the term principal of heat exchange with the help of this activity.
The cold object the hot object enclosed in one box of heat-resistant material.
- What changes will occur in the two objects when temperature flows from those objects?
- Which principle can show that the energy exchange takes place between two objects only when kept in isolated system?
Numerical Problem.
What could be the final temperature of a mixture of 100 g of water at 90 °C and 600g of water at 20°C.
Which of the following substances (A, B and C) has the highest specific beat?
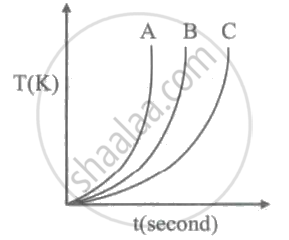
Conductors have generally high specific heat capacities and insulators have low specific heat capacities.
A metal ball of heat capacity 50J/°C loses 2000 J of heat. By how much will its temperature fall?
Thermal capacities of substances A and B are same. If mass of A is more than mass of B then:
Which substance will have more specific heat capacity?
