Advertisements
Advertisements
प्रश्न
In a photoelectric experiment, the collector plate is at 2.0 V with respect to the emitter plate made of copper (φ = 4.5 eV). The emitter is illuminated by a source of monochromatic light of wavelength 200 nm. Find the minimum and maximum kinetic energy of the photoelectrons reaching the collector.
उत्तर
Given:-
Work function of copper, ϕ = 4.5 eV,
Wavelength of monochromatic light, λ = 200 nm
From Einstein's photoelectric equation, kinetic energy,
`K = E - phi = (hc)/lambda - phi`,
where, h = Planck's constant
c = speed of light
`therefore K = 1242/200 - 4.5`
`= 6.21 - 4.5 = 1.71 "eV"`
Thus, at least 1.7 eV is required to stop the electron. Therefore, minimum kinetic energy will be 2 eV.
It is given that electric potential of 2 V is required to accelerate the electron. Therefore, maximum kinetic energy
`= (2 + 1.7) "eV" = 3.7 "eV"`
APPEARS IN
संबंधित प्रश्न
In an experiment on the photoelectric effect, the slope of the cut-off voltage versus the frequency of incident light is found to be 4.12 × 10−15 Vs. Calculate the value of Planck’s constant.
The work function for a certain metal is 4.2 eV. Will this metal give photoelectric emission for incident radiation of wavelength 330 nm?
In an accelerator experiment on high-energy collisions of electrons with positrons, a certain event is interpreted as annihilation of an electron-positron pair of total energy 10.2 BeV into two γ-rays of equal energy. What is the wavelength associated with each γ-ray? (1BeV = 109 eV)
Briefly explain the three observed features which can be explained by Einstein’s photoelectric equation.
Define the terms (i) ‘cut-off voltage’ and (ii) ‘threshold frequency’ in relation to the phenomenon of photoelectric effect.
Using Einstein’s photoelectric equation shows how the cut-off voltage and threshold frequency for a given photosensitive material can be determined with the help of a suitable plot/graph.
Is p − E/c valid for electrons?
The frequency and intensity of a light source are doubled. Consider the following statements.
(A) The saturation photocurrent remains almost the same.
(B) The maximum kinetic energy of the photoelectrons is doubled.
A non-monochromatic light is used in an experiment on photoelectric effect. The stopping potential
The electric field at a point associated with a light wave is `E = (100 "Vm"^-1) sin [(3.0 xx 10^15 "s"^-1)t] sin [(6.0 xx 10^15 "s"^-1)t]`.If this light falls on a metal surface with a work function of 2.0 eV, what will be the maximum kinetic energy of the photoelectrons?
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
A monochromatic light source of intensity 5 mW emits 8 × 1015 photons per second. This light ejects photoelectrons from a metal surface. The stopping potential for this setup is 2.0 V. Calculate the work function of the metal.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
A small metal plate (work function φ) is kept at a distance d from a singly-ionised, fixed ion. A monochromatic light beam is incident on the metal plate and photoelectrons are emitted. Find the maximum wavelength of the light beam, so that some of the photoelectrons may go round the ion along a circle.
Use Einstein’s photoelectric equation to show how from this graph,
(i) Threshold frequency, and (ii) Planck’s constant can be determined.
How does one explain the emission of electrons from a photosensitive surface with the help of Einstein’s photoelectric equation?
Choose the correct answer from given options
Photons of frequency v are incident on the surface of two metals A and B of threshold frequency 3/4 v and 2/3 v, respectively. The ratio of maximum kinetic energy of electrons emitted from A to that from B is
Each photon has the same speed but different ______.
The wavelength of a photon needed to remove a proton from a nucleus which is bound to the nucleus with 1 MeV energy is nearly ______.
There are materials which absorb photons of shorter wavelength and emit photons of longer wavelength. Can there be stable substances which absorb photons of larger wavelength and emit light of shorter wavelength.
A student performs an experiment on photoelectric effect, using two materials A and B. A plot of Vstop vs ν is given in Figure.
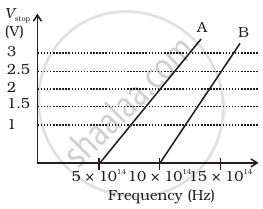
- Which material A or B has a higher work function?
- Given the electric charge of an electron = 1.6 × 10–19 C, find the value of h obtained from the experiment for both A and B.
Comment on whether it is consistent with Einstein’s theory:
The photon emitted during the de-excitation from the first excited level to the ground state of a hydrogen atom is used to irradiate a photocathode in which the stopping potential is 5 V. Calculate the work function of the cathode used.
