Advertisements
Advertisements
प्रश्न
The work function of caesium metal is 2.14 eV. When light of frequency 6 × 1014 Hz is incident on the metal surface, photoemission of electrons occurs. What is the
- maximum kinetic energy of the emitted electrons,
- Stopping potential, and
- maximum speed of the emitted photoelectrons?
उत्तर
Work function of caesium metal, `phi_0` = 2.14 eV
Frequency of light, v = 6.0 × 1014 Hz
(a) The maximum kinetic energy is given by the photoelectric effect as:
K = hv − `phi_0`
Where,
h = Planck’s constant = 6.626 × 10−34 Js
∴ K = `(6.626 xx 10^-34 xx 6 xx 10^14)/(1.6 xx 10^(-19)) - 2.14`
= 2.485 − 2.140
= 0.345 eV
Hence, the maximum kinetic energy of the emitted electrons is 0.345 eV.
(b) For stopping potential V0, we can write the equation for kinetic energy as:
K = eV0
∴ `V_0 = K/e`
= `(0.345 xx 1.6 xx 10^(-19))/(1.6 xx 10^(-19))`
= 0.345 V
Hence, the stopping potential of the material is 0.345 V.
(c) Maximum speed of the emitted photoelectrons = v
Hence, the relation for kinetic energy can be written as:
K = `1/2 mv^2`
Where,
m = Mass of an electron = 9.1 × 10−31 kg
`v^2 = (2K)/m`
= `(2 xx 0.345 xx 1.6 xx 10^(-19))/(9.1 xx 10^(-31))`
= 0.1104 × 1012
∴ v = 3.323 × 105 m/s
= 332.3 km/s
Hence, the maximum speed of the emitted photoelectrons is 332.3 km/s.
APPEARS IN
संबंधित प्रश्न
Why should gases be insulators at ordinary pressures and start conducting at very low pressures?
How will the thermionic current vary if the filament current is increased?
Would you prefer a material with a high work-function or a low work-function to be used as a cathode in a diode?
An isolated metal sphere is heated to a high temperature. Will it become positively charged due to thermionic emission?
A diode value is connected to a battery and a load resistance. The filament is heated, so that a constant current is obtained in the circuit. As the cathode continuously emits electrons, does it become more and more positively charged?
Why does thermionic emission not take place in non-conductors?
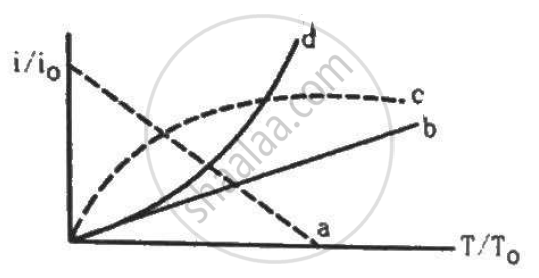
Let i0 be the thermionic current from a metal surface when the absolute temperature of the surface is T0. The temperature is slowly increased and the thermionic current is measured as a function of temperature. Which of the following plots may represent the variation in (i/i0) against (T/T0)?
The wavelength λe of an electron and λp of a photon of same energy E are related by
If a light of wavelength 330 nm is incident on a metal with work function 3.55 eV, the electrons are emitted. Then the wavelength of the wave associated with the emitted electron is (Take h = 6.6 × 10–34 Js)
Why do metals have a large number of free electrons?
Define the work function of a metal. Give its unit.
What do you mean by electron emission? Explain briefly various methods of electron emission.
In which case is electron emission from a metal not known?
Photoelectric emission is observed from a metallic surface for frequencies ν1 and ν2 of the incident light (ν1 > ν2). If the maximum value of kinetic energy of the photoelectrons emitted in the two cases are in the ration 1 : n then the threshold frequency of the metallic surface is ______.
Give an example each of a metal from which photoelectric emission takes place when irradiated by
- UV light
- visible light.
Name the factors on which photoelectric emission from a surface depends.
