Advertisements
Advertisements
प्रश्न
The photoelectric cut-off voltage in a certain experiment is 1.5 V. What is the maximum kinetic energy of photoelectrons emitted?
उत्तर
Photoelectric cut-off voltage, V0 = 1.5 V
The maximum kinetic energy of the emitted photoelectrons is given as:
Ke = eV0
Where,
e = Charge on an electron = 1.6 × 10−19 C
∴ Ke = 1.6 × 10−19 × 1.5
= 2.4 × 10−19 J
Therefore, the maximum kinetic energy of the photoelectrons emitted in the given experiment is 2.4 × 10−19 J.
APPEARS IN
संबंधित प्रश्न
Light of intensity 10−5 W m−2 falls on a sodium photo-cell of surface area 2 cm2. Assuming that the top 5 layers of sodium absorb the incident energy, estimate time required for photoelectric emission in the wave-picture of radiation. The work function for the metal is given to be about 2 eV. What is the implication of your answer?
Every metal has a definite work function. Why do all photoelectrons not come out with the same energy if incident radiation is monochromatic? Why is there an energy distribution of photoelectrons?
Can a photon be deflected by an electric field? Or by a magnetic field?
The threshold wavelength of a metal is λ0. Light of wavelength slightly less than λ0 is incident on an insulated plate made of this metal. It is found that photoelectrons are emitted for some time and after that the emission stops. Explain.
Two photons of
The equation E = pc is valid
A point source causes photoelectric effect from a small metal plate. Which of the following curves may represent the saturation photocurrent as a function of the distance between the source and the metal?

Photoelectric effect supports quantum nature of light because
(a) there is a minimum frequency below which no photoelectrons are emitted
(b) the maximum kinetic energy of photoelectrons depends only on the frequency of light and not on its intensity
(c) even when the metal surface is faintly illuminated the photoelectrons leave the surface immediately
(d) electric charge of the photoelectrons is quantised
A totally reflecting, small plane mirror placed horizontally faces a parallel beam of light, as shown in the figure. The mass of the mirror is 20 g. Assume that there is no absorption in the lens and that 30% of the light emitted by the source goes through the lens. Find the power of the source needed to support the weight of the mirror.
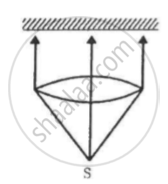
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
The work function of a metal is 2.5 × 10−19 J. (a) Find the threshold frequency for photoelectric emission. (b) If the metal is exposed to a light beam of frequency 6.0 × 1014 Hz, what will be the stopping potential?
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
The figure is the plot of stopping potential versus the frequency of the light used in an experiment on photoelectric effect. Find (a) the ratio h/e and (b) the work function.
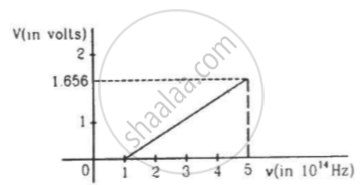
Define the terms "stopping potential' and 'threshold frequency' in relation to the photoelectric effect. How does one determine these physical quantities using Einstein's equation?
Define the term: stopping potential in the photoelectric effect.
Consider a metal exposed to light of wavelength 600 nm. The maximum energy of the electron doubles when light of wavelength 400 nm is used. Find the work function in eV.
Two monochromatic beams A and B of equal intensity I, hit a screen. The number of photons hitting the screen by beam A is twice that by beam B. Then what inference can you make about their frequencies?
Consider a 20 W bulb emitting light of wavelength 5000 Å and shining on a metal surface kept at a distance 2 m. Assume that the metal surface has work function of 2 eV and that each atom on the metal surface can be treated as a circular disk of radius 1.5 Å.
- Estimate no. of photons emitted by the bulb per second. [Assume no other losses]
- Will there be photoelectric emission?
- How much time would be required by the atomic disk to receive energy equal to work function (2 eV)?
- How many photons would atomic disk receive within time duration calculated in (iii) above?
- Can you explain how photoelectric effect was observed instantaneously?
The work function for a metal surface is 4.14 eV. The threshold wavelength for this metal surface is ______.
Why it is the frequency and not the intensity of the light source that determines whether the emission of photoelectrons will occur or not? Explain.
How would the stopping potential for a given photosensitive surface change if the frequency of the incident radiation were increased? Justify your answer.
