Advertisements
Advertisements
प्रश्न
What is the action of the following on ethyl bromide:
moist silver oxide
उत्तर
\[\ce{2C2H5Br + Ag2O->C2H5 - O - C2H5 + 2AgBr}\]
\[\ce{Ag2O + H2O -> 2AgOH}\]
\[\ce{AgOH + C2H5Br -> C2\underset{\text{Ethanol}}{H5OH} + AgBr }\]
APPEARS IN
संबंधित प्रश्न
Given reasons: C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH2Br + KCN ->[aq.ethanol]}\]
The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
SN1 reactions are accompanied by racemization in optically active alkyl halides.
AgCN reacts with haloalkanes to form isocyanide. Haloalkanes react with KCN to form alkyl cyanides as the main product. Why?
Which of the following is optically inactive?
Which of the following is a primary halide?
Optically active isomers but not mirror images are called ____________.
SN2 mechanism proceeds through intervention of ____________.
Which of the following is the correct order of decreasing SN2 reactivity?
Which of the following compounds is optically active?
Among the following, the dissociation constant is highest for:
SN1 reaction of alkyl halides lead to ___________.
Which of the following compounds will give a racemic mixture on nucleophilic substitution by OH ion?
1-Bromoethane, 1-Bromopropane, 1-Bromobutane, Bromobenzene
Complete the following analogy:
Same molecular formula but different structures: A : : Non superimposable mirror images: B
Which of the following statements are correct about this reaction?
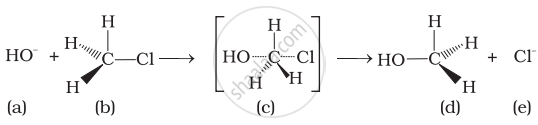
(i) The given reaction follows SN2 mechanism.
(ii) (b) and (d) have opposite configuration.
(iii) (b) and (d) have same configuration.
(iv) The given reaction follows SN1 mechanism.
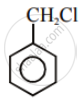
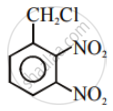
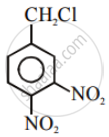
Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution:
| (I) | 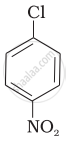 |
| (II) | 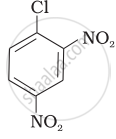 |
| (III) | 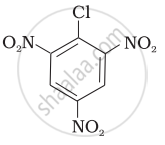 |
Which one is the correct order of nucleophilic strength (pKa) of following nucleophiles?
Which one of the following compounds is more reactive towards SN1 reaction?
Give reason for the following:
The product formed during SN1 reaction is a racemic mixture.
In which reaction mechanism carbocation is formed?
Which of the following compounds will show retention in configuration on nucleophile substitution by OH− ion?
Optical activity of an enantiomeric mixture is +12.6° and the specific rotation of (+) isomer is +30°. The optical purity is ______ %.
The decreasing order of reactivity of the following compounds towards nucleophilic substitution (SN2) is ______.
Arrange the following compounds in increasing order of reactivity towards SN2 reaction.
2-Bromopentane, 1-Bromopentane, 2-Bromo-2-methylbutane
Convert bromoethane to propanamine.
The following questions are case-based questions. Read the passage carefully and answer the questions that follow:
|
Nucleophilic Substitution: Influences of solvent polarity: The reaction rate (SN2) of 2-bromopropane and NaOH in ethanol containing 40% water is twice slower than in absolute ethanol. Hence the level of solvent polarity has an influence on both SN1 and SN2 reactions but with different results. Generally speaking, a weak polar solvent is favourable for SN2 reaction, while a strong polar solvent is favourable for SN1. Generally speaking, the substitution reaction of tertiary haloalkane is based on SN1 mechanism in solvents with a strong polarity (for example ethanol containing water). |
Answer the following questions:
(a) Why racemisation occurs in SN1? (1)
(b) Why is ethanol less polar than water? (1)
(c) Which one of, the following in each pair is more reactive towards SN2 reaction? (2)
(i) CH3 – CH2 – I or CH3CH2 – Cl
(ii)
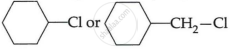
OR
(c) Arrange the following in the increasing order of their reactivity towards SN1 reactions: (2)
(i) 2-Bromo-2-methylbutane, 1-Bromo-pentane, 2-Bromo-pentane
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3- methylbutane
Discuss the mechanism of alkaline hydrolysis of methyl bromide.