Advertisements
Advertisements
Question
A certain gas 'X' occupies a volume of 100 cm3 at S.T.P. and weighs 0.5 g. Find its relative molecular mass.
Solution
At STP 100 cm3 of gas weights = 0.5 g
1 cm3 of a gas will weigh = `0.5/100`g
∴ At STP 22400 cm3 of gas will weight = `0.5/100 xx 22400`
= 112 g
APPEARS IN
RELATED QUESTIONS
Give balanced chemical equations for the following conversions A, B, and C:
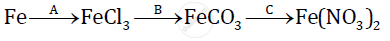
Aluminium carbide reacts with water according to the following equation :
`Al_4C_3 + 12H_2O-> 4Al(OH)_3 + 3CH_4`
1)What mass of aluminium hydroxide is formed from 12g of aluminium carbide?
2) What volume of methane at s.t.p. is obtained from 12g of aluminium carbide?
[Relatively molecular weight of `Al_4Cl_3 = 144; Al(OH)_3 = 78]`
The usefulness of a fertilizer depends upon percentage of nitrogen present in it. Find which of the following is a better fertilizer:
(a) Ammonium nitrate [NH4NO3]
(b) Ammonium phosphate [(NH4)3PO4 (N=14,H=1,O=16,P=31)
When excess lead nitrate solution was added to a solution of sodium sulphate, 15.1g of lead sulphate was precipitated. What mass of sodium sulphate was present in the original solution?
Na2SO4 + Pb(NO3)2 → PbSO4 + 2NaNO3
(H = 1, C = 12, O = 16, Na = 23, S = 32, Pb = 207)
Calculate the percentage of phosphorous in the fertilizer superphosphate, Ca(H2PO4)2. [Ca = 40, H =1, P =31, O = 16] (Correct to 1 decimal place)
Calculate the percentage of nitrogen in aluminium nitride. [Al = 27, N = 14]
A gaseous hydrocarbon contains 82.76% of carbon. Given that its vapour density is 29, find its molecular formula.
[C = 12, H = 1]
Calculate the relative molecular mass of:
Potassium chlorate
A gas cylinder can hold 1 kg of hydrogen at room temperature and pressure. What mass of carbon dioxide can it hold under similar conditions of temperature and pressure?
Ammonia burns in oxygen and the combustion, in the presence of a catalyst, may be represented by;
\[\ce{2NH3 + 2 1/2O2 -> 2NO + 3H2O}\] [H = 1, N = 14, O = 16]
What mass of steam is produced when 1.5 g of nitrogen monoxide is formed?
