Advertisements
Advertisements
Question
A human body excretes (removes by waste discharge, sweating, etc.) certain materials by a law similar to radioactivity. If technetium is injected in some form in a human body, the body excretes half the amount in 24 hours. A patient is given an injection containing 99Tc. This isotope is radioactive with a half-life of 6 hours. The activity from the body just after the injection is 6 μCi. How much time will elapse before the activity falls to 3 μCi?
Solution
Given :
Time taken by the body to excrete half the amount, t1 = 24 hours
Half-life of radioactive isotope, t2 = 6 hours
Initial activity, A0 = 6 μCi
Let after time t, activity of the sample be A.
Half-life period (`T_"1/2"`) is given by
`T_"1/2" = (t_1t_2)/(t_1 + t_2) = (24 xx 6)/(24 + 6)`
= `(24 xx 6)/30` = 4.8 h
Activity (A) at time t is given by
`therefore A = A_0/2^(t/T_"1/2"`
⇒ `3"μCi" = (6"μCi")/2^(t/4.8)`
⇒ `(6"μCi")/2^(t/4.8) = 3`
⇒ t = 4.8 h
APPEARS IN
RELATED QUESTIONS
Write nuclear reaction equation for β−-decay of `""_83^210"Bi"`.
Write nuclear reaction equation for β+-decay of `""_6^11"C"`.
Define ‘activity’ of a radioactive material and write its S.I. units.
The sequence of stepwise decay of a radioactive nucleus is
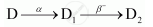
If the atomic number and mass number of D2 are 71 and 176 respectively, what are their corresponding values of D?
State the law of radioactive decay. hence derive the relation N = Noe-λt . Represent it graphically.
The half-life of 199Au is 2.7 days. (a) Find the activity of a sample containing 1.00 µg of 198Au. (b) What will be the activity after 7 days? Take the atomic weight of 198Au to be 198 g mol−1.
Radioactive 131I has a half-life of 8.0 days. A sample containing 131I has activity 20 µCi at t = 0. (a) What is its activity at t = 4 days? (b) What is its decay constant at t = 4.0 days?
The count rate from a radioactive sample falls from 4.0 × 106 per second to 1.0 × 106per second in 20 hours. What will be the count rate 100 hours after the beginning?
The selling rate of a radioactive isotope is decided by its activity. What will be the second-hand rate of a one month old 32P(t1/2 = 14.3 days) source if it was originally purchased for 800 rupees?
In an agricultural experiment, a solution containing 1 mole of a radioactive material (t1/2= 14.3 days) was injected into the roots of a plant. The plant was allowed 70 hours to settle down and then activity was measured in its fruit. If the activity measured was 1 µCi, what per cent of activity is transmitted from the root to the fruit in steady state?
The count rate of nuclear radiation coming from a radiation coming from a radioactive sample containing 128I varies with time as follows.
| Time t (minute): | 0 | 25 | 50 | 75 | 100 |
| Ctount rate R (109 s−1): | 30 | 16 | 8.0 | 3.8 | 2.0 |
(a) Plot In (R0/R) against t. (b) From the slope of the best straight line through the points, find the decay constant λ. (c) Calculate the half-life t1/2.
4 × 1023 tritium atoms are contained in a vessel. The half-life of decay tritium nuclei is 12.3 y. Find (a) the activity of the sample, (b) the number of decay in the next 10 hours (c) the number of decays in the next 6.15 y.
238U decays to 206Pb with a half-life of 4.47 × 109 y. This happens in a number of steps. Can you justify a single half for this chain of processes? A sample of rock is found to contain 2.00 mg of 238U and 0.600 mg of 206Pb. Assuming that all the lead has come from uranium, find the life of the rock.
`""_83^212"Bi"` can disintegrate either by emitting an α-particle of by emitting a β−-particle. (a) Write the two equations showing the products of the decays. (b) The probabilities of disintegration α-and β-decays are in the ratio 7/13. The overall half-life of 212Bi is one hour. If 1 g of pure 212Bi is taken at 12.00 noon, what will be the composition of this sample at 1 P.m. the same day?
A sample contains a mixture of 108Ag and 110Ag isotopes each having an activity of 8.0 × 108 disintegration per second. 110Ag is known to have larger half-life than 108Ag. The activity A is measured as a function of time and the following data are obtained.
| Time (s) |
Activity (A) (108 disinte- grations s−1) |
Time (s) |
Activity (A 108 disinte-grations s−1) |
| 20 40 60 80 100 |
11.799 9.1680 7.4492 6.2684 5.4115 |
200 300 400 500 |
3.0828 1.8899 1.1671 0.7212 |
(a) Plot ln (A/A0) versus time. (b) See that for large values of time, the plot is nearly linear. Deduce the half-life of 110Ag from this portion of the plot. (c) Use the half-life of 110Ag to calculate the activity corresponding to 108Ag in the first 50 s. (d) Plot In (A/A0) versus time for 108Ag for the first 50 s. (e) Find the half-life of 108Ag.
Plot a graph showing the variation of undecayed nuclei N versus time t. From the graph, find out how one can determine the half-life and average life of the radioactive nuclei.
Half-life of a certain radioactive material is 8 hours.
Find the disintegration constant of this material.
A nucleus with Z = 92 emits the following in a sequence:
α, β‾, β‾, α, α, α, α, α, β‾, β‾, α, β+, β+, α
Then Z of the resulting nucleus is ______.
