Advertisements
Advertisements
Question
A sample contains a mixture of 108Ag and 110Ag isotopes each having an activity of 8.0 × 108 disintegration per second. 110Ag is known to have larger half-life than 108Ag. The activity A is measured as a function of time and the following data are obtained.
| Time (s) |
Activity (A) (108 disinte- grations s−1) |
Time (s) |
Activity (A 108 disinte-grations s−1) |
| 20 40 60 80 100 |
11.799 9.1680 7.4492 6.2684 5.4115 |
200 300 400 500 |
3.0828 1.8899 1.1671 0.7212 |
(a) Plot ln (A/A0) versus time. (b) See that for large values of time, the plot is nearly linear. Deduce the half-life of 110Ag from this portion of the plot. (c) Use the half-life of 110Ag to calculate the activity corresponding to 108Ag in the first 50 s. (d) Plot In (A/A0) versus time for 108Ag for the first 50 s. (e) Find the half-life of 108Ag.
Solution
(a) Activity, A0 = 8 × 108 dis/sec
(i)
(ii)
(iii)
(iv)
(v)
(vi)
(vii)
(viii)
(ix)
The required graph is given below.
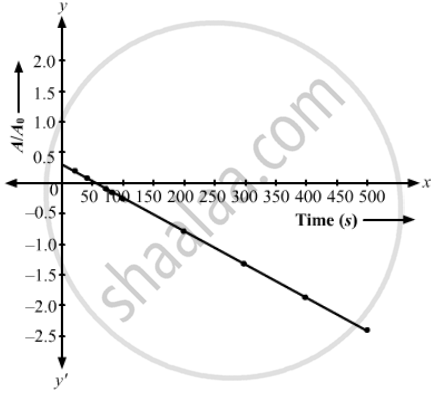
(b) Half-life of 110Ag = 24.4 s
(c) Half-life of 110Ag,
Decay constant,
⇒
Activity ,
=
=
(d)
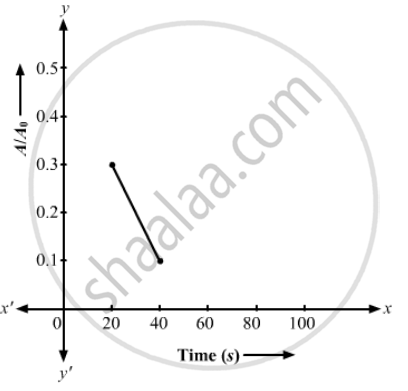
(e) The half-life period of
APPEARS IN
RELATED QUESTIONS
The half life of a certain radioactive material against \u0003α-decay is 100 days. After how much time, will the undecayed fraction of the material be 6.25%?
Write nuclear reaction equation for β−-decay of
Write nuclear reaction equation for β+-decay of
Write nuclear reaction equation for electron capture of
A radioactive nucleus has a decay constant λ = 0.3465 (day)–1. How long would it take the nucleus to decay to 75% of its initial amount?
Define ‘activity’ of a radioactive material and write its S.I. units.
The sequence of stepwise decay of a radioactive nucleus is
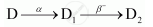
If the atomic number and mass number of D2 are 71 and 176 respectively, what are their corresponding values of D?
The half-life of 199Au is 2.7 days. (a) Find the activity of a sample containing 1.00 µg of 198Au. (b) What will be the activity after 7 days? Take the atomic weight of 198Au to be 198 g mol−1.
A certain sample of a radioactive material decays at the rate of 500 per second at a certain time. The count rate falls to 200 per second after 50 minutes. (a) What is the decay constant of the sample? (b) What is its half-life?
The half-life of 226Ra is 1602 y. Calculate the activity of 0.1 g of RaCl2 in which all the radium is in the form of 226Ra. Taken atomic weight of Ra to be 226 g mol−1 and that of Cl to be 35.5 g mol−1.
The selling rate of a radioactive isotope is decided by its activity. What will be the second-hand rate of a one month old 32P(t1/2 = 14.3 days) source if it was originally purchased for 800 rupees?
The count rate of nuclear radiation coming from a radiation coming from a radioactive sample containing 128I varies with time as follows.
| Time t (minute): | 0 | 25 | 50 | 75 | 100 |
| Ctount rate R (109 s−1): | 30 | 16 | 8.0 | 3.8 | 2.0 |
(a) Plot In (R0/R) against t. (b) From the slope of the best straight line through the points, find the decay constant λ. (c) Calculate the half-life t1/2.
238U decays to 206Pb with a half-life of 4.47 × 109 y. This happens in a number of steps. Can you justify a single half for this chain of processes? A sample of rock is found to contain 2.00 mg of 238U and 0.600 mg of 206Pb. Assuming that all the lead has come from uranium, find the life of the rock.
A charged capacitor of capacitance C is discharged through a resistance R. A radioactive sample decays with an average-life τ. Find the value of R for which the ratio of the electrostatic field energy stored in the capacitor to the activity of the radioactive sample remains constant in time.
In a gamma ray emission from nucleus :
Copy and complete the following table for a radioactive element whose half-life is 10 minutes. Assume that you have 30g of this element at t = 0.

Plot a graph showing the variation of undecayed nuclei N versus time t. From the graph, find out how one can determine the half-life and average life of the radioactive nuclei.
Half-life of a certain radioactive material is 8 hours.
Find the disintegration constant of this material.
A nucleus with Z = 92 emits the following in a sequence:
α, β‾, β‾, α, α, α, α, α, β‾, β‾, α, β+, β+, α
Then Z of the resulting nucleus is ______.
