Advertisements
Advertisements
Question
An antifriction alloy made up of antimony with tin and copper, which is extensively used in machine bearings is called _______.
(A) Duralumin
(B) Babbitt metal
(C) Spiegeleisen
(D) Amalgam
Solution
(B) Babbitt metal
APPEARS IN
RELATED QUESTIONS
The elements of 3d transition series are given as: Sc Ti V Cr Mn Fe Co
Answer the following: Write the element which shows maximum number of oxidation states. Give reason.
How would you account for the irregular variation of ionization enthalpies (first and second) in the first series of the transition elements?
To what extent do the electronic configurations decide the stability of oxidation states in the first series of the transition elements? Illustrate your answer with examples.
What are the characteristics of the transition elements and why are they called transition elements?
In what way is the electronic configuration of the transition elements different from that of the non-transition elements?
For M2+/M and M3+/M2+ systems, the EΘ values for some metals are as follows:
| Cr2+/Cr | −0.9 V |
| Mn2+/Mn | −1.2 V |
| Fe2+/Fe | −0.4 V |
| Cr3/Cr2+ | −0.4 V |
| Mn3+/Mn2+ | +1.5 V |
| Fe3+/Fe2+ | +0.8 V |
Use this data to comment upon:
The stability of Fe3+ in acid solution as compared to that of Cr3+ or Mn3+.
How would you account for the following:
Of the d4 species, Cr2+ is strongly reducing while manganese (III) is strongly oxidising.
Write the formula of an oxo-anion of Chromium (Cr) in which it shows the oxidation state equal to its group number
Give reasons: E° value for the Mn3+/Mn2+ couple is much more positive than that for Fe3+/Fe2+.
The transition metals show _________ character because of the presence of unpaired· electrons and Cu+ is ____________ because of its electronic configuration is [Ar]3d10
Maximum magnetic moment is shown by ____________.
Generally transition elements form coloured salts due to the presence of unpaired electrons. Which of the following compounds will be coloured in solid-state?
Transition elements show magnetic moment due to spin and orbital motion of electrons. Which of the following metallic ions have almost same spin only magnetic moment?
(i) \[\ce{Co^{2+}}\]
(ii) \[\ce{Cr^{2+}}\]
(iii) \[\ce{Mn^{2+}}\]
(iv) \[\ce{Cr^{3+}}\]
Although \[\ce{Cr^3+}\] and \[\ce{Co^2+}\] ions have same number of unpaired electrons but the magnetic moment of \[\ce{Cr^3+}\] is 3.87 B.M. and that of \[\ce{Co^2+}\] is 4.87 B.M. Why?
A solution of \[\ce{KMnO4}\] on reduction yields either a colourless solution or a brown precipitate or a green solution depending on pH of the solution. What different stages of the reduction do these represent and how are they carried out?
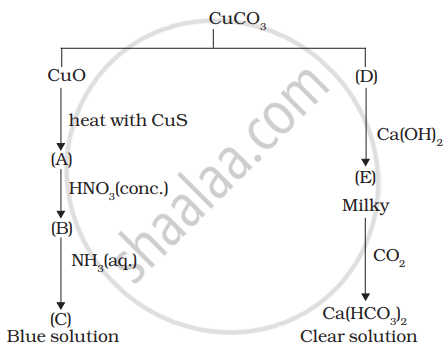
Identify A to E and also explain the reactions involved.
On the basis of the figure given below, answer the following questions:
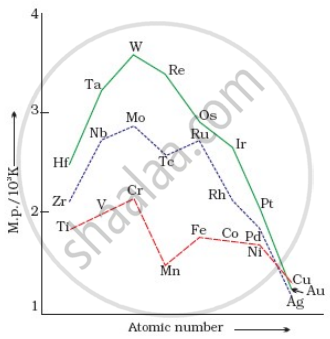
- Why Manganese has lower melting point than Chromium?
- Why do transition metals of 3d series have lower melting points as compared to 4d series?
- In the third transition series, identify and name the metal with the highest melting point.
Which of the following species has maximum magnetic momentum?
Which of the following ions will exhibit colour in aqueous solution?
A complex in which dsp2 hybridisation takes place is
Why is the `"E"_(("V"^(3+)//"V"^(2+)))^"o"` value for vanadium comparatively low?
The number of terminal oxygen atoms present in the product B obtained from the following reactions is:
\[\ce{FeCr2O4 + Na2CO3 + O2 -> A + Fe2O3 + CO2}\]
\[\ce{A + H^+ -> B + H2O + Na^+}\]
The disproportionation of \[\ce{MnO^{2-}_4}\] in acidic medium resulted in the formation of two manganese compounds A and B. If the oxidation state of Mn in B is smaller than that of A, then the spin-only magnetic moment (µ) value of B in BM is ______. (Nearest integer)
Which of the following transition metals shows +1 and +2 oxidation states?
Which of the following ions has the electronic configuration 3d6?
(Atomic number: Mn = 25, Co = 27, Ni = 28)
Account for the following:
Copper has an exceptionally positive `"E"_("M"^(2+)//"M")^0` value.
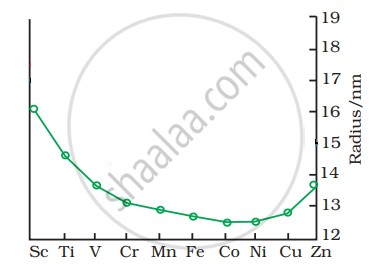
The trend of which property is represented by the following graph?
Write the ionic equation for reaction of KI with acidified KMnO4.
Account for the following:
Zirconium (Zr) and Hafnium (Hf) are difficult to separate.
