Advertisements
Advertisements
Question
Answer the following.
Explain reverse osmosis.
Solution
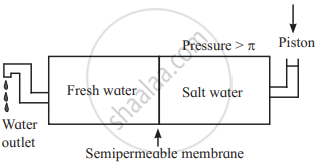
i. If a pressure larger than the osmotic pressure is applied to the solution side, then pure solvent from the solution passes into pure solvent side through the semipermeable membrane. This phenomenon is called reverse osmosis.
ii. For example, consider fresh water salt water separated by a semipermeable membrane. When the pressure larger than the osmotic pressure of a solution is applied to solution, pure water from salty water passes into fresh pure water through the membrane. Thus, the direction of osmosis can be reversed by applying a pressure larger than the osmotic pressure.
iii. The schematic set up for reverse osmosis is as follows:
APPEARS IN
RELATED QUESTIONS
Determine the osmotic pressure of a solution prepared by dissolving 2.5 × 10−2 g of K2SO4 in 2L of water at 25°C, assuming that it is completely dissociated.
(R = 0.0821 L atm K−1 mol−1, Molar mass of K2SO4 = 174 g mol−1)
Which of the following is not a colligative property?
Blood cells are isotonic with 0.9% sodium chloride solution. What happens if we place blood cells in a solution containing
(i) 1.2% sodium chloride solution?
(ii) 0.4% sodium chloride solution?
A solution containing 15 g urea (molar mass = 60 g mol–1) per litre of solution in water has the same osmotic pressure (isotonic) as a solution of glucose (molar mass = 180 g mol–1) in water. Calculate the mass of glucose present in one litre of its solution.
Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving 1.0 g of polymer of molar mass 185,000 in 450 mL of water at 37°C.
Determine the amount of CaCl2 (i = 2.47) dissolved in 2.5 litre of water such that its osmotic pressure is 0.75 atm at 27°C.
Determine the osmotic pressure of a solution prepared by dissolving 25 mg of K2SO4 in 2 liter of water at 25°C, assuming that it is completely dissociated.
Define osmotic pressure.
Calculate the mass of NaCl (molar mass = 58.5 g mol−1) to be dissolved in 37.2 g of water to lower the freezing point by 2°C, assuming that NaCl undergoes complete dissociation. (Kf for water = 1.86 K kg mol−1)
Define the following term:
isotonic solution
Define the following term:
hypertonic solution
Define the following term:
hypotonic solution
Choose the most correct option.
In calculating osmotic pressure the concentration of solute is expressed in _______.
Choose the most correct option.
The osmotic pressure of blood is 7.65 atm at 310 K. An aqueous solution of glucose isotonic with blood has the percentage (by volume)________.
Answer the following in one or two sentences.
What is osmotic pressure?
Answer the following in one or two sentences.
A solution concentration is expressed in molarity and not in molality while considering osmotic pressure. Why?
Answer the following.
What are isotonic and hypertonic solutions?
Answer the following.
A solvent and its solution containing a nonvolatile solute are separated by a semipermeable membrane. Does the flow of solvent occur in both directions? Comment giving a reason.
An aqueous solution of a certain organic compound has a density of 1.063 g mL-1 , osmotic pressure of 12.16 atm at 25 °C and a freezing point of 1.03 °C. What is the molar mass of the compound?
Explain the osmotic pressure of a solution with the help of a thistle tube.
Explain the phenomenon of osmosis.
20 g of a substance were dissolved in 500 mL of water and the osmotic pressure of the solution was found to be 600 mm of mercury at 15°C. The molecular weight of the substance is:
The average osmotic pressure of human blood is 7.8 bar at 37°C. What is the concentration of an aqueous NaCl solution that could be used in the blood stream?
Osmotic pressure of a solution is 0.0821 atm at a temperature of 300 K. The concentration in moles/litre will be:
At a given temperature, osmotic pressure of a concentrated solution of a substance ______.
Isotonic solutions must have the same:
(i) solute
(ii) density
(iii) elevation in boiling point
(iv) depression in freezing point
In isotonic solutions:
(i) Solute and solvent both are same.
(ii) Osmotic pressure is same.
(iii) Solute and solvent may or may not be same.
(iv) Solute is always same solvent may be different.
Give an example of a material used for making semipermeable membrane for carrying out reverse osmosis.
Match the items given in Column I and Column II.
| Column I | Column II |
| (i) Saturated solution | (a) Solution having same osmotic pressure at a given temperature as that of given solution. |
| (ii) Binary solution | (b) A solution whose osmotic pressure is less than that of another. |
| (iii) Isotonic solution | (c) Solution with two components. |
| (iv) Hypotonic solution | (d) A solution which contains maximum amount of solute that can be dissolved in a given amount of solvent at a given temperature. |
| (v) Solid solution | (e) A solution whose osmotic pressure is more than that of another. |
| (vi) Hypertonic solution | (f) A solution in solid phase. |
How can you remove the hard calcium carbonate layer of the egg without damaging its semiprermiable membrane? Can this egg be inserted into a bottle with a narrow neck without distorting its shape? Explain the process involved.
Osmotic pressure of a solution increases if
Which of the following colligative property can provide molar mass of proteins (or polymers or colloids) with greatest precision?
Blood cells retain their normal shape in solution which are
In Isotonic solution
The vapour pressure of water is 12.3 k pa at 300 k. Calculated the vapour pressure of molal solution in it.
Osmotic pressure of a solution containing 2 g dissolved protein per 300 cm3 of solution is 20 mm of Hg at 27°C. The molecular mass of protein is ______.
The following solutions were prepared by dissolving 10 g of glucose \[\ce{(C6H12O6)}\] in 250 ml of water (P1), 10 g of urea \[\ce{(CH4N2O)}\] in 250 ml of water (P2) and 10 g of sucrose \[\ce{(C12H22O11}\]) in 250 ml of water (P3). The right option for the decreasing order of osmotic pressure of these solutions is ______
Derive an expression to calculate molar mass of non-volatile solute by osmotic pressure measurement.
Assertion (A) : Osmotic pressure is a colligative property.
Reason (R) : Osmotic pressure is proportional to the molality.
Determine the osmotic pressure of a solution prepared by dissolving 2.32 × 10−2 g of K2SO4 in 2L of solution at 25°C assuming that K2SO4 is completely dissociated.
(R = 0.082 L atm K−1 mol, Molar mass K2SO4 = 174 g mol−1)
A solution containing 10 g glucose has osmotic pressure 3.84 atm. If 10 g more glucose is added to the same solution, what will be its osmotic pressure? (Temperature remains constant)
Define osmotic pressure (π).
Define reverse osmosis.
Name the four colligative properties that are oftently used for determination of molecular mass.
How will you determine molar mass of solute from osmotic pressure?
