Advertisements
Advertisements
Question
At what temperature is the root mean square speed of an atom in an argon gas cylinder equal to the rms speed of a helium gas atom at – 20 °C? (atomic mass of Ar = 39.9 u, of He = 4.0 u).
Solution 1
Temperature of the helium atom, THe = –20°C= 253 K
Atomic mass of argon, MAr = 39.9 u
Atomic mass of helium, MHe = 4.0 u
Let, (vrms)Ar be the rms speed of argon.
Let (vrms)He be the rms speed of helium.
The rms speed of argon is given by:
`(v_"rms")_"Ar" = sqrt((3RT_"Ar")/M_"Ar")` .... (1)
Where,
R is the universal gas constant
TAr is temperature of argon gas
The rms speed of helium is given by:
`(v_"rms")_"He" = sqrt((3RT_"He")/M_"He")` ... (ii)
It is given that
(vrms)Ar = (vrms)He
`sqrt((3RT_"Ar")/ M_"Ar")` = `sqrt((3RT_"He")/M_"He")`
`T_"Ar"/M_"Ar" = T_"He"/M_"He"`
`T_"Ar" = T_"He"/M_"He" xx M_"Ar"`
`= 253/4 xx 39.9`
= 2523.675 = 2.52 × 103 K
Therefore, the temperature of the argon atom is 2.52 × 103 K.
Solution 2
Let C and C’ be the rms velocity of argon and a helium gas atoms at temperature T K and T K respectively
Here, M = 39.9; M’ = 4.0; T =?; T = -20 + 273 = 253 K
Now, `C = sqrt((3RT)/M
)= sqrt((3RT)/39.9)` and `C' = sqrt((3RT')/M') = sqrt(3R xx 253)/4`
Since C =C'
Therefore `sqrt((3RT)/39.9) = sqrt(3Rxx253)/4` or `T = (39.9 xx 253)/4 = 2523.7 K`
APPEARS IN
RELATED QUESTIONS
The figure shows the plot of PV/T versus Pfor 1.00×10–3 kg of oxygen gas at two different temperatures.
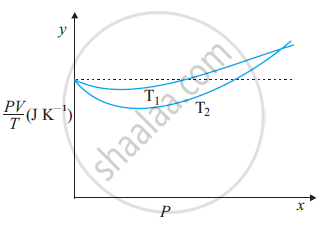
(a) What does the dotted plot signify?
(b) Which is true: T1 > T2 or T1 < T2?
(c) What is the value of PV/T where the curves meet on the y-axis?
(d) If we obtained similar plots for 1.00 ×10–3 kg of hydrogen, would we get the same value of PV/T at the point where the curves meet on the y-axis? If not, what mass of hydrogen yields the same value of PV/T (for low pressure high temperature region of the plot)? (Molecular mass of H2 = 2.02 u, of O2 = 32.0 u, R = 8.31 J mo1–1 K–1.)
Oxygen is filled in a closed metal jar of volume 1.0 × 10−3 m3 at a pressure of 1.5 × 105Pa and temperature 400 K. The jar has a small leak in it. The atmospheric pressure is 1.0 × 105 Pa and the atmospheric temperature is 300 K. Find the mass of the gas that leaks out by the time the pressure and the temperature inside the jar equalise with the surrounding.
Correct the following statement:
0°C is equal to zero Kelvin.
Name or state the following:
An equation used in chemical calculations which gives a simultaneous effect of changes of temperature and pressure on the volume of a given mass of dry gas
Give reason for the following:
Temperature remaining constant the product of the vol. & the press, of a given mass of dry gas is a constant.
Show that for monoatomic gas the ratio of the two specific heats is 5:3.
The equation of state for 2g of oxygen at a pressure 'P' and temperature 'T', when occupying a volume 'V' will be ______.
Estimate the average thermal energy of a helium atom at the temperature on the surface of the Sun (6000 K).
At room temperature, a diatomic gas is found to have an r.m.s. speed of 1930 ms-1. The gas is ______.
For a wave, y = 0.0002 sin`[2pi(110"t"-x/3)+pi/3]` is travelling in a medium. The energy per unit volume being transferred by wave if density of medium is 1.5 kg/m3, is ______.
