Advertisements
Advertisements
Question
Calculate mass defect and binding energy per nucleon of `"_10^20 Ne`, given
Mass of `"_10^20 Ne= 19.992397` u
Mass of `"_0^1H = 1.007825` u
Mass of `"_0^1n = 1.008665` u
Solution
Mass defect (Δm) = Mass of nucleons − Mass of nucleus
= (10 × 1.007825 + 10 × 1.008665)u - 19.992397u
= 0.172503u
Binding energy ΔE = 0.1725034 × 931 MeV
= 160.600 MeV
Binding energy per nucleon `(ΔE)/A = 160.600/20`
= 8.03 MeV
RELATED QUESTIONS
Consider the fission of `""_92^238"U"` by fast neutrons. In one fission event, no neutrons are emitted and the final end products, after the beta decay of the primary fragments, are `""_58^140"Ce"` and `""_44^99"Ru"`. Calculate Q for this fission process. The relevant atomic and particle masses are
`"m"(""_92^238"U")` = 238.05079 u
`"m"(""_58^140"Ce")` = 139.90543 u
`"m"(""_44^99"Ru")` = 98.90594 u
Define half-life of a radioactive substance
Use this graph to explain the release of energy in both the processes of nuclear fusion and fission.
In which of the following decays the atomic number decreases?
(a) α-decay
(b) β+-decay
(c) β−-decay
(d) γ-decay
Binding energy per nucleon for helium nucleus (2 He) is 7.0 MeV Find value of mass defect for helium nucleus
Sketch a graph showing the variation of binding energy per nucleon of a nucleus with its mass number.
The figure shows the plot of binding energy (BE) per nucleon as a function of mass number A. The letters A, B, C, D, and E represent the positions of typical nuclei on the curve. Point out, giving reasons, the two processes (in terms of A, B, C, D, and E ), one of which can occur due to nuclear fission and the other due to nuclear fusion.
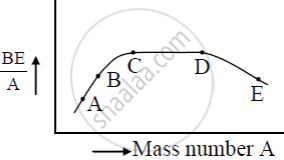
Answer the following question.
Draw the curve showing the variation of binding energy per nucleon with the mass number of nuclei. Using it explains the fusion of nuclei lying on the ascending part and fission of nuclei lying on the descending part of this curve.
Explain the release of energy in nuclear fission and fusion on the basis of binding energy per nucleon curve.
What is binding energy of nucleus?
