Advertisements
Advertisements
Question
Explain the important aspects of resonance with reference to the `"CO"_3^(2-)` ion.
Solution 1

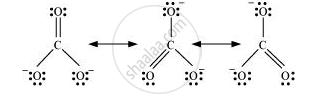
Resonance in `"CO"_3^(2-)`, I, II and III represent the three canonical forms.
- In these structures, the position of nuclei are same.
- All three forms have almost equal energy.
- Same number of paired and impaired electrons differ only in their position.
Solution 2
According to experimental findings, all carbon to oxygen bonds in `"CO"_3^(2-)` are equivalent. Hence, it is inadequate to represent `"CO"_3^(2-)` ion by a single Lewis structure having two single bonds and one double bond.
Therefore, carbonate ion is described as a resonance hybrid of the following structures
APPEARS IN
RELATED QUESTIONS
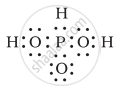
H3PO3 can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing H3PO3? If not, give reasons for the same.
 |
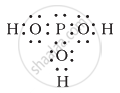 |
| (1) | (2) |
Write the resonance structure for SO3.
Write the resonance structures for NO2.
Write the resonance structures for `"NO"_3^(-)`.
Which of the following species has tetrahedral geometry?
Which of the following statements are not correct?
(i) \[\ce{NaCl}\] being an ionic compound is a good conductor of electricity in the solid state.
(ii) In canonical structures there is a difference in the arrangement of atoms.
(iii) Hybrid orbitals form stronger bonds than pure orbitals.
(iv) \[\ce{VSEPR}\] Theory can explain the square planar geometry of \[\ce{XeF4}\].
Explain why \[\ce{CO^{2–}3}\] ion cannot be represented by a single Lewis structure. How can it be best represented?
Draw the resonating structure of ozone molecule
Draw the resonating structure of nitrate ion
Resonance structures can be written for ______.
The acceptable resonating structures of the following molecule are:
\[\begin{array}{cc}
\ce{CH3 - CH = C - \overset{\bullet\bullet}{\underset{\bullet\bullet}{O}} - CH2 - CH3}\\
|\phantom{......}\\
\ce{:N}\phantom{.......}\\
\phantom{}/\phantom{...}\backslash\phantom{......}\\
\phantom{}\ce{H3C}\phantom{.....}\ce{CH3}\phantom{......}
\end{array}\]
| (x) | \[\begin{array}{cc} \ce{CH3 - \overset{\overset{Θ}{\bullet\bullet}}{C}H - C - \overset{\bullet\bullet}{\underset{\bullet\bullet}{O}} - CH2 - CH3}\\ ||\phantom{......}\\ \ce{N^⊕}\phantom{.....}\\ \phantom{}/\phantom{...}\backslash\phantom{......}\\ \phantom{}\ce{H3C}\phantom{.....}\ce{CH3}\phantom{......} \end{array}\] |
| (y) | \[\begin{array}{cc} \ce{CH3 - \overset{\overset{Θ}{\bullet\bullet}}{C}H - C = \overset{⊕}{\underset{\bullet\bullet}{O}} - CH2 - CH3}\\ |\phantom{......}\\ \ce{:N}\phantom{.......}\\ \phantom{}/\phantom{...}\backslash\phantom{......}\\ \phantom{}\ce{H3C}\phantom{.....}\ce{CH3}\phantom{......} \end{array}\] |
| (z) | \[\begin{array}{cc} \ce{CH3 - \overset{⊕}{C}H - C - \overset{\bullet\bullet}{\underset{\bullet\bullet}{O}} - CH2 - CH3}\\ ||\phantom{......}\\ \ce{N^Θ}\phantom{.....}\\ \phantom{}/\phantom{...}\backslash\phantom{......}\\ \phantom{}\ce{H3C}\phantom{.....}\ce{CH3}\phantom{......} \end{array}\] |
| (w) | \[\begin{array}{cc} \ce{CH3 - \overset{⊕}{C}H - C = \overset{Θ}{O} - CH2 - CH3}\\ |\phantom{......}\\ \ce{:N}\phantom{.......}\\ \phantom{}/\phantom{...}\backslash\phantom{......}\\ \phantom{}\ce{H3C}\phantom{.....}\ce{CH3}\phantom{......} \end{array}\] |
