Advertisements
Advertisements
Question
Explain why \[\ce{CO^{2–}3}\] ion cannot be represented by a single Lewis structure. How can it be best represented?
Solution
The carbonate ion \[\ce{CO^{2–}3}\] can be best represented by its resonating structures. The carbonate ion cannot be represented by a single Lewis structure because the three carbon-oxygen bond lengths are the same. This cannot be shown by a single Lewis structure. For showing the similar lengths of all the carbon to oxygen bonds three hybrid structures are constructed which are in resonance with each other.
APPEARS IN
RELATED QUESTIONS
Explain the important aspects of resonance with reference to the `"CO"_3^(2-)` ion.
H3PO3 can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing H3PO3? If not, give reasons for the same.
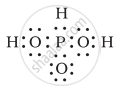 |
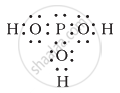 |
| (1) | (2) |
Write the resonance structure for SO3.
Write the resonance structures for NO2.
Write the resonance structures for `"NO"_3^(-)`.
Which of the following species has tetrahedral geometry?
Which of the following statements are not correct?
(i) \[\ce{NaCl}\] being an ionic compound is a good conductor of electricity in the solid state.
(ii) In canonical structures there is a difference in the arrangement of atoms.
(iii) Hybrid orbitals form stronger bonds than pure orbitals.
(iv) \[\ce{VSEPR}\] Theory can explain the square planar geometry of \[\ce{XeF4}\].
Draw the resonating structure of ozone molecule
Resonance structures can be written for ______.
The acceptable resonating structures of the following molecule are:
\[\begin{array}{cc}
\ce{CH3 - CH = C - \overset{\bullet\bullet}{\underset{\bullet\bullet}{O}} - CH2 - CH3}\\
|\phantom{......}\\
\ce{:N}\phantom{.......}\\
\phantom{}/\phantom{...}\backslash\phantom{......}\\
\phantom{}\ce{H3C}\phantom{.....}\ce{CH3}\phantom{......}
\end{array}\]
| (x) | \[\begin{array}{cc} \ce{CH3 - \overset{\overset{Θ}{\bullet\bullet}}{C}H - C - \overset{\bullet\bullet}{\underset{\bullet\bullet}{O}} - CH2 - CH3}\\ ||\phantom{......}\\ \ce{N^⊕}\phantom{.....}\\ \phantom{}/\phantom{...}\backslash\phantom{......}\\ \phantom{}\ce{H3C}\phantom{.....}\ce{CH3}\phantom{......} \end{array}\] |
| (y) | \[\begin{array}{cc} \ce{CH3 - \overset{\overset{Θ}{\bullet\bullet}}{C}H - C = \overset{⊕}{\underset{\bullet\bullet}{O}} - CH2 - CH3}\\ |\phantom{......}\\ \ce{:N}\phantom{.......}\\ \phantom{}/\phantom{...}\backslash\phantom{......}\\ \phantom{}\ce{H3C}\phantom{.....}\ce{CH3}\phantom{......} \end{array}\] |
| (z) | \[\begin{array}{cc} \ce{CH3 - \overset{⊕}{C}H - C - \overset{\bullet\bullet}{\underset{\bullet\bullet}{O}} - CH2 - CH3}\\ ||\phantom{......}\\ \ce{N^Θ}\phantom{.....}\\ \phantom{}/\phantom{...}\backslash\phantom{......}\\ \phantom{}\ce{H3C}\phantom{.....}\ce{CH3}\phantom{......} \end{array}\] |
| (w) | \[\begin{array}{cc} \ce{CH3 - \overset{⊕}{C}H - C = \overset{Θ}{O} - CH2 - CH3}\\ |\phantom{......}\\ \ce{:N}\phantom{.......}\\ \phantom{}/\phantom{...}\backslash\phantom{......}\\ \phantom{}\ce{H3C}\phantom{.....}\ce{CH3}\phantom{......} \end{array}\] |
