Advertisements
Advertisements
Question
Explain the meaning of heat and work with suitable examples.
Solution
Meaning of heat: When an object at a higher temperature is placed in contact with another object at a lower temperature, there will be a spontaneous flow of energy from the object at a higher temperature to the one at a lower temperature. This energy is called heat. This process of energy transfer from higher temperature objects to lower temperature objects is called heating. Due to the flow of heat sometimes the temperature of the body will increase or sometimes it may not increase.
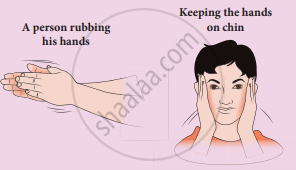
Meaning of work: When you rub your hands against each other the temperature of the hand's increases. You have done some work on your hands by rubbing. The temperature of the hands increases due to this work. Now if you place your hands on the cheek, the temperature of the cheek increases. This is because the hands are at a higher temperature than the cheek. In the above example, the temperature of the hands is increased due to work, and the temperature of the cheek is increased due to heat transfer from the hands to the chin. It is shown in the Figure. By doing work on the system, the temperature in the system will increase and sometimes may not. Like heat, work is also not a quantity and through the work-energy is transferred to the system. So we cannot use the word ‘the object contains more work’ or ‘less work’.
Either the system can transfer energy to the surrounding by doing work on surrounding or the surrounding may transfer energy to the system by doing work on the system. For the transfer of energy from one body to another body through the process of work, they need not be at different temperatures.
APPEARS IN
RELATED QUESTIONS
A metal ball cools from 64 °C to 50 °C in 10 minutes and to 42 °C in next 10 minutes. The ratio of rates of fall of temperature during the two intervals is _______.
Can the bulb of a thermometer be made of an adiabatic wall?
Which of the curves in the following figure represents the relation between Celsius and Fahrenheit temperatures?
Is heat a conserved quantity?
Which of the following pairs of physical quantities may be represented in the same unit?
A circular hole of diameter 2.00 cm is made in an aluminium plate at 0°C. What will be the diameter at 100°C? α for aluminium = 2.3 × 10–5 °C–1.
A steel rod of length 1 m rests on a smooth horizontal base. If it is heated from 0°C to 100°C, what is the longitudinal strain developed?
In hot summer after a bath, the body’s __________.
Give reasons for the following:
Hot metal ball of 80° C is dipped into water of 80°C. The ball will not contract.
Two identical beakers A and B contain equal volumes of two different liquids at 60°C each and is left to cool down. Liquid in A has a density of 8 × 102 kg/m3 and specific heat of 2000 J kg-1 K-1 while the liquid in B has a density of 103 kg m-3 and specific heat of 4000 J kg-1 K-1. Which of the following best describes their temperature versus time graph schematically? (assume the emissivity of both the beakers to be the same.)
