Advertisements
Advertisements
Question
Find the odd man out:
Camphor, Ammonium Chloride, Naphthalene balls, Sugar
Solution
Sugar
Camphor, ammonium chloride, naphthalene balls are volatile, while sugar is a non-volatile substance.
APPEARS IN
RELATED QUESTIONS
A solution of a substance ‘X’ is used for white washing.
- Name the substance ‘X’ and write its formula.
- Write the reaction of the substance ‘X’ named in (i) above with water.
Gas A, which is the major cause of global warming, combines with hydrogen oxide B in nature in the presence of an environmental factor C and a green material D to form a six carbon organic compounds E and a gas F. The gas F is necessary for breathing.
(a) What is gas A?
(b) What is the common name of B?
(c) What do you think could be C?
(d) What is material D? Where is it found?
(e) Name the organic compound E.
(f) What is gas F? Name the natural process during which it is released.
What type of chemical reaction take place when a magnesium wire is burnt in air?
What type of chemical reaction take place when ammonia and hydrogen chloride are mixed?
What type of chemical reaction is represented by the following equation?
A + B → C
what tpye of reaction is the following:
2K + Cl → 2KCl
What type of reaction is represented by the following equation?
CaO + CO2 → CaCO3
Define a combination reaction.
Give one example of a combination reaction which is also exothermic.
Consider the following equation of the chemical reaction of a metal M:
4M + 3O2 → 2M2O3
This equation represents:
(a) combination reaction as well as reduction reaction
(b) decomposition reaction as well as oxidation reaction
(c) oxidation reaction as well as displacement reaction
(d) combination reaction as well as oxidation reaction
When hydrogen burns in oxygen, water is formed and when water is electrolysed, then hydrogen and oxygen are produced. What type of reaction takes place:
(a) in the first case?
(b) in the second case
What happens during a chemical reaction ?
Give one example each of which illustrates the following characteristics of a chemical reaction:
change of colour
What do you observe when solid lead nitrate is heated?
What do you observe when when dilute sulphuric acid is added to barium chloride solution ?
Fill in the blank
A reaction in which two or more substances combine to form a single substance is called a ............ reaction.
Fill in the blank
The chemical reaction between hydrogen and chlorine is a ................ reaction
(a) What is double displacement reaction? Explain with an example.
(b) A small amount of quick lime is added to water in a beaker.
(i) Name and define the type of reaction that has taken place.
(ii) Write balanced chemical equation for the above reaction and the chemical name of the product formed.
(iii) List two main observations of this reaction.
Define: Chemical combination reaction
Calcium oxide reacts vigorously with water to produce slaked lime.
\[\ce{CaO{(s)} + H2O(l) -> Ca(OH)2(aq)}\]
This reaction can be classified as:
(A) Combination reaction
(B) Exothermic reaction
(C) Endothermic reaction
(D) Oxidation reaction
Classify the following reaction into different type:
CaO(s) + H2O(l) → Ca(OH)2(aq)
State why a direct combination reaction is called a – ‘synthesis reaction’.
Give a balanced equation for the following type of reaction:
A reaction of direct combination i.e. synthesis in which twp gases combine to give another gas – which turns lime water milky.
The number of products formed in a synthesis reaction is ______
Define a combination reaction. Give one example of an exothermic combination reaction.
In which of the following chemical equations, the abbreviations represent the correct states of the reactants and products involved at reaction temperature?
The respiration process during which glucose undergoes slow combustion by combining with oxygen in the cells of our body to produce energy is a kind of:
The chemical reaction between copper and oxygen can be categorized as ______.
The Salt Story
From: The New Indian Express 9 March 2021
The salt pans in Marakkanam, a port town about 120 km from Chennai are the third largest producer of salt in Tamil Nadu. Separation of salt from water is a laborious process and the salt obtained is used as raw materials for manufacture of various sodium compounds.
One such compound is Sodium hydrogen carbonate, used in baking, as an antacid and in soda acid fire extinguishers.
The table shows the mass of various compounds obtained when 1litre of sea water is evaporated.
| COMPOUND | FORMULA | MASS OF SOLID PRESENT /g |
| Sodium Chloride | NaCl | 28.0 |
| Magnesium Chloride | MgCl2 | 8.0 |
| Magnesium Sulphate | MgSO4 | 6.0 |
| Calcium Sulphate | CaSO4 | 2.0 |
| Calcium Carbonate | CaCO3 | 1.0 |
| TOTAL AMOUNT OF SALT OBTAINED | 45.0 | |
Which compound in the table reacts with acids to release carbon dioxide?
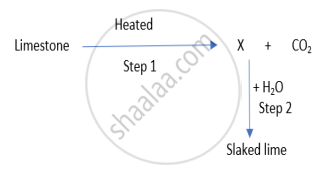
Identify the correct option from the given table which represents the type of reactions occurring in step 1 and step 2.
Balance the following chemical equation and identify the type of chemical reaction.
`"CaO"("s") + "SiO"_2("s") -> "CaSio"_3("s")`
Read the text below and answer the questions that follow:
A small amount of hydrochloric acid was taken in a test tube. The test tube was heated. A glass rod was dipped in the ammonia solution and held on the top of the test tube. A white smoke was seen emanating from the tip of the glass rod.
- What must have happened?
- Which colour of gas is formed?
- Write the chemical equation for the reaction.
A clear solution of slaked lime is made by dissolving Ca(OH)2 in an excess of water. This solution is left exposed to air. The solution slowly goes milky as a faint white precipitate forms. Explain why a faint white precipitate forms, support your response with the help of a chemical equation.
A metal ribbon 'X' bums in oxygen with a dazzling white flame forming a white ash 'Y'. The correct description of X, Y and the type of reaction is:
