Advertisements
Advertisements
Question
In the two tetrahedral structures of dichromate ion, ______.
Options
4 Cr–O bonds are equivalent in length
6 Cr–O bonds are equivalent in length
All Cr–O bonds are equivalent in length
All Cr–O bonds are non-equivalent
Solution
In the two tetrahedral structures of dichromate ion, 6 Cr–O bonds are equivalent in length.
Explanation:
The Dichromate di-anion is made up of two tetrahedral molecules that share oxygen at their common corner. The structure of the chromate ion is tetrahedral. Due to resonance, the six terminal bonds in the dichromate ion have the same bond length. As a result, all six Cr–O bonds are equivalent.
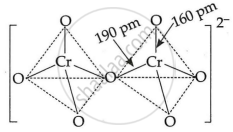
6 Cr–O bonds are equivalent
APPEARS IN
RELATED QUESTIONS
Complete the following equation:
\[\ce{2MnO4- + 6H+ + 5NO2- ->}\]
Complete the following equation : MnO4- + 8H+ + 5e- →
Indicate the steps in the preparation of K2Cr2O7 from chromite ore.
Account for the following :
Manganese shows maximum number of oxidation states in 3d series.
Write the ionic equation showing the oxidation of Fe(II) salt by acidified dichromate solutions.
Using IUPAC norms write the formulae of Potassium trioxalatochromate (III)
Zinc carbonate is precipitated from zinc sulphate solution by the addition of ___________.
Complete the reaction mentioning all the products formed:
\[\ce{Cr2O^{2-}7 + 3H2S + 8H^+ ->}\]
Indicate the steps in the preparation of \[\ce{K2Cr2O2}\] from chromite ore.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
