Advertisements
Advertisements
Question
Plot a graph to show the variation of stopping potential with frequency of incident radiation in relation to photoelectric effect.
Solution 1
Due to increase in frequency there is increase in K.E. of electron.
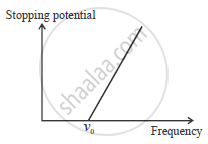
So stopping potential increases with increase in frequency of incides ralation.
Solution 2
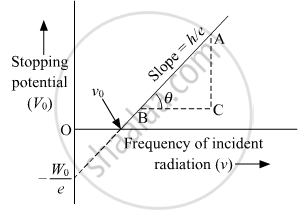
Solution 3

RELATED QUESTIONS
If light of wavelength 412.5 nm is incident on each of the metals given below, which ones will show photoelectric emission and why?
| Metal | Work Function (eV) |
| Na | 1.92 |
| K | 2.15 |
| Ca | 3.20 |
| Mo | 4.17 |
Define the term "cut off frequency" in photoelectric emission. The threshod frequency of a metal is f. When the light of frequency 2f is incident on the metal plate, the maximum velocity of photo-electrons is v1. When the frequency of the incident radiation is increased to 5f, the maximum velocity of phto-electrons is v2. Find the ratio v1 : v2.
Visible light has wavelengths in the range of 400 nm to 780 nm. Calculate the range of energy of the photons of visible light.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
In an experiment on photoelectric effect, light of wavelength 400 nm is incident on a cesium plate at the rate of 5.0 W. The potential of the collector plate is made sufficiently positive with respect to the emitter, so that the current reaches its saturation value. Assuming that on average, one out of every 106 photons is able to eject a photoelectron, find the photocurrent in the circuit.
A silver ball of radius 4.8 cm is suspended by a thread in a vacuum chamber. Ultraviolet light of wavelength 200 nm is incident on the ball for some time during which light energy of 1.0 × 10−7 J falls on the surface. Assuming that on average, one photon out of every ten thousand is able to eject a photoelectron, find the electric potential at the surface of the ball, assuming zero potential at infinity. What is the potential at the centre of the ball?
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
Work function of aluminium is 4.2 eV. If two photons each of energy 2.5 eV are incident on its surface, will the emission of electrons take place? Justify your answer.
In the case of a photo electric effect experiment, explain the following facts, giving reasons.
The wave theory of light could not explain the existence of the threshold frequency.
The electromagnetic theory of light failed to explain ______.
In photoelectric effect, the photoelectric current
An increase in the intensity of the radiation causing photo-electric emission from a surface does not affect the maximum K.E. of the photoelectrons. Explain.
