Advertisements
Advertisements
Question
Pressure versus volume graph for a real gas and an ideal gas are shown in figure. Answer the following questions on the basis of this graph.
(i) Interpret the behaviour of real gas with respect to ideal gas at low pressure.
(ii) Interpret the behaviour of real gas with respect to ideal gas at high pressure.
(iii) Mark the pressure and volume by drawing a line at the point where real gas behaves as an ideal gas.
Solution
(i) At low pressure, the curve of real gas coincides with that of ideal gas, this shows that the deviation of behaviour of real gas with respect to ideal gas is small or negligible.
(ii) At high pressure, the curve of real gas is far apart from ideal gas, this shows that the deviation of behaviour of real gas with respect to ideal gas is large.
(iii) The pressure p1 and volume V1 are the point where real gas behaves as an ideal gas. 
APPEARS IN
RELATED QUESTIONS
Which of the following diagrams correctly describes the behaviour of a fixed mass of an ideal gas? (T is measured in K)
In what way real gases differ from ideal gases.
Can a Van der Waals gas with a = 0 be liquefied? explain.
Explain whether a gas approaches ideal behavior or deviates from ideal behaviour if it is compressed to a smaller volume at a constant temperature.
A plot of volume (V) versus temperature (T) for a gas at constant pressure is a straight line passing through the origin. The plots at different values of pressure are shown in Figure. Which of the following order of pressure is correct for this gas?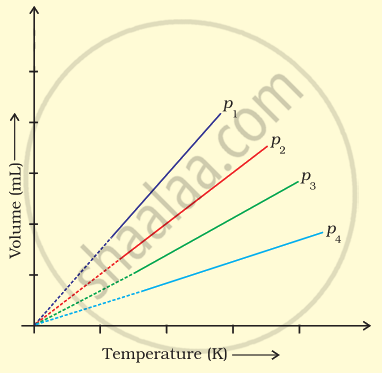
Under which of the following two conditions applied together, a gas deviates most from the ideal behaviour?
(i) Low pressure
(ii) High pressure
(iii) Low temperature
(iv) High temperature
Compressibility factor, Z, of a gas is given as Z = `(pV)/(nRT)`. What is the value of Z for an ideal gas?
Match the following graphs of ideal gas with their co-ordinates:
| Graphical representation | x and y co-ordinates |
(i)  |
(a) pV vs. V |
(ii) 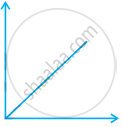 |
(b) p vs. V |
(iii) 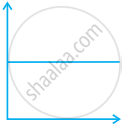 |
(c) p vs. `1/V` |
Isotherms of carbon dioxide gas are shown in figure. Mark a path for changing gas into liquid such that only one phase (i.e., either a gas or a liquid) exists at any time during the change. Explain how the temperature, volume and pressure should be changed to carry out the change.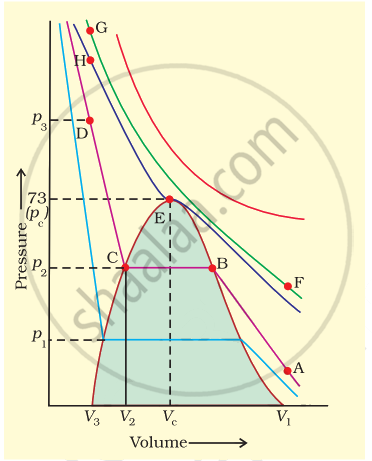
In van der Waal's equation for the real gas, the expression for the net force of attraction amongst the gas molecules is given by:
