Advertisements
Advertisements
Question
The container shown in figure has two chambers, separated by a partition, of volumes V1 = 2.0 litre and V2 = 3.0 litre. The chambers contain µ1 = 4.0 and µ2 = 5.0 moles of a gas at pressures p1 = 1.00 atm and p2 = 2.00 atm. Calculate the pressure after the partition is removed and the mixture attains equilibrium.
| V1 | V2 |
| µ1, p1 | µ2 |
| p2 |
Solution
Consider the diagram,
| p1V1 | p2V2 |
| µ1 | µ2 |
Given, V1 = 2.0 L, V2 = 3.0 L
µ1 = 4.0 mol, µ2 = 5.0 mol
p1 = 1.00 atm, p2 = 2.00 atm
For chamber 1, p1, V1 = µ1RT1
For chamber 2, p2, V2 = µ2RT2
When the partition is removed the gases get mixed without any loss of energy. The mixture Now attains a common equilibrium pressure and the total volume of the system is the sum of the volume of individual chambers V1 and V2.
So, µ = µ1 + µ2, V = V1 + V2
From the kinetic theory of gases,
For `l` mole `pV = 2/3 E` ......`[(E = "Translational"),("Kinetic energy")]`
For `µ_1` moles, `p_1V_1 = 2/3 µ_1E_1`
For `µ_2` moles, `p_2V_2 = 2/3 µ_2E_2`
The total energy is `(µ_1E_1 + µ_2E_2) = 3/2 (p_1V_1 + p_2V_2)`
From the above relation, `pV = 2/3 E_"total" = 2/3 µE_"per mole"`
`p(V_1 + V_2) = 2/3 xx 3/2 (p_1V_1 + p_2V_2)`
`p = (p_1V_1 + p_2V_2)/(V_1 + V_2)`
= `((1.00 + 2.0 + 2.00 xx 3.0)/(2.0 + 3.0))` atm
= `8.0/5.0`
= 1.60 atm
APPEARS IN
RELATED QUESTIONS
The energy of a given sample of an ideal gas depends only on its
Consider the quantity \[\frac{MkT}{pV}\] of an ideal gas where M is the mass of the gas. It depends on the
Find the number of molecules in 1 cm3 of an ideal gas at 0°C and at a pressure of 10−5mm of mercury.
Use R = 8.31 J K-1 mol-1
A sample of 0.177 g of an ideal gas occupies 1000 cm3 at STP. Calculate the rms speed of the gas molecules.
A rigid container of negligible heat capacity contains one mole of an ideal gas. The temperature of the gas increases by 1° C if 3.0 cal of heat is added to it. The gas may be
(a) helium
(b) argon
(c) oxygen
(d) carbon dioxide
The ratio of the molar heat capacities of an ideal gas is Cp/Cv = 7/6. Calculate the change in internal energy of 1.0 mole of the gas when its temperature is raised by 50 K (a) keeping the pressure constant (b) keeping the volume constant and (c) adiaba
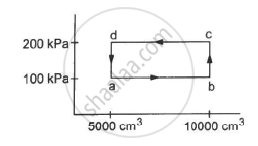
Half mole of an ideal gas (γ = 5/3) is taken through the cycle abcda, as shown in the figure. Take `"R" = 25/3"J""K"^-1 "mol"^-1 `. (a) Find the temperature of the gas in the states a, b, c and d. (b) Find the amount of heat supplied in the processes ab and bc. (c) Find the amount of heat liberated in the processes cd and da.
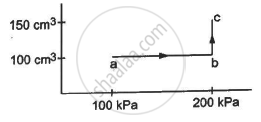
An ideal gas (γ = 1.67) is taken through the process abc shown in the figure. The temperature at point a is 300 K. Calculate (a) the temperatures at b and c (b) the work done in the process (c) the amount of heat supplied in the path ab and in the path bcand (d) the change in the internal energy of the gas in the process.
A cubic vessel (with faces horizontal + vertical) contains an ideal gas at NTP. The vessel is being carried by a rocket which is moving at a speed of 500 ms–1 in vertical direction. The pressure of the gas inside the vessel as observed by us on the ground ______.
When an ideal gas is compressed adiabatically, its temperature rises: the molecules on the average have more kinetic energy than before. The kinetic energy increases ______.
- because of collisions with moving parts of the wall only.
- because of collisions with the entire wall.
- because the molecules gets accelerated in their motion inside the volume.
- because of redistribution of energy amongst the molecules.
