Advertisements
Advertisements
Question
The reaction between acetic acid and sodium carbonate is shown in the following figure.
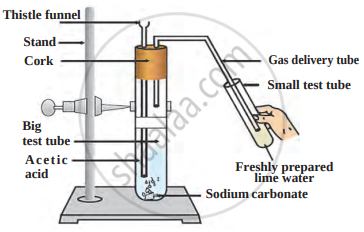
Answer the questions with the help of a diagram.
- Which gas does come out as effervescence in the big test tube?
- What is the colour change in the lime water present in the small test tube?
- Write the related reaction.
Solution
- Carbon dioxide gas.
- Lime water turns milky.
- \[\ce{2CH3COOH (aq) + Na2CO3 (g) -> CH3COONa (aq) + H2O (l) + CO2 (g)}\]
APPEARS IN
RELATED QUESTIONS
The molecular formula of acetic acid is _________ .
(a) CH3COOH
(b) CH3 – CH3
(c) C6H6
(d) C2H4
Write three different chemical reactions showing the conversion of ethanoic acid to sodium ethanoate. Write balanced chemical equation in each case. Write the name of the reactants and the products other ethanoic acid and sodium ethanoate in each case.
A student puts a drop of reaction mixture of a saponification reaction first a blue litmus paper and then on a red litmus paper. He may observe that:
(a) There is no change in the blue litmus paper and the red litmus paper turns white.
(b) There is no change in the red litmus paper and the blue litmus paper turns red.
(c) There is no change in the blue litmus paper and the red litmus paper turns blue.
(d) No change in colour is observed in both the litmus papers
Consider the following comments about saponification reactions:
I. Heat is evolved in these reactions.
II. For quick precipitation of soap, sodium chloride is added to the reaction mixtures.
III. Saponification reactions are a special kind of neutralisation reactions.
IV. Soaps are basic salts of long-chain fatty acids.
The correct comments are
(a) I, II and III
(b) II, III and IV
(c) I, II and IV
(d) Only I and IV
Complete the following chemical equations : C2H5OH`("Conc."H_2SO_4)/(443K)`>
When zinc powder is added to acetic acid ______________
(a) the mixture becomes warm
(b) a gas is evolved
(c) the colour of the mixture becomes yellow
(d) a solid settles at the bottom
Complete the following equation:
`CH_3 COOH + CH_2 H_5 OH` 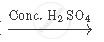
Fill in the following blank with a suitable word:
The organic acid present in vinegar is ______.
How would you distinguish experimentally between an alcohol and carboxylic acid on the basis of a chemical property?
How does ethanoic acid react with sodium hydrogen carbonate? Give equation of the reaction which takes place.
What happens when ethanoic acid reacts with sodium hydroxide? Write equation of the reaction involved.
A student takes 2 mL acetic acid in a dry test tube and adds a pinch of sodium hydrogen carbonate to it. He makes the following observations:
I. A colourless and odourless gas evolves with a brisk effervescence.
II. The gas turns lime water milky when passed through it.
III. The gas burns with an explosion when a burning splinter is brought near it.
IV. The gas extinguishes the burning splinter that is brought near it.
The correct observations are:
(A) I, II, and III
(B) II, III and IV
(C) III, IV and I
(D) IV, I and II
Fill in the blank with appropriate word/words.
Denatured alcohol is a mixture of _____ and _______
Two statements are given - one labelled Assertion (A) and the other labelled Reason (R).
Assertion (A): Esterification is a process in which a sweet-smelling substance is produced.
Reason (R): When esters react with sodium hydroxide an alcohol and sodium salt of carboxylic acid are obtained.
Anita added a drop each of diluted acetic acid and diluted hydrochloric acid on pH paper and compared the colors. Which of the following is the correct conclusion?
Give the balanced chemical equation of the following reaction:
Oxidation of ethanol by acidified potassium dichromate.
How is ethanol to ethanoic acid an oxidation reaction different from the reaction in which ethanol burns in the presence of oxygen?
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
Give the balanced chemical equation of the reaction.
Oxidation of ethanol by acidified potassium dichromate.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
