Advertisements
Advertisements
Question
Why does compound (A) given below not form an oxime?
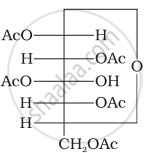
(A)
Solution
Glucose pentaacetate (structure A) does not have a free –OH group at C, and therefore, cannot be converted to the open chain form to give a free –CHO group and hence it does not form the oxime.
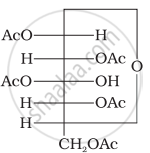
(Structure A)
APPEARS IN
RELATED QUESTIONS
Glucose on reaction with HI gives n-hexane. What does it suggest about the structure of glucose?
The following compound can be called as:
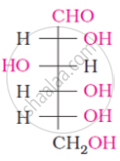
What is the most abundant organic compound on earth?
Glucose is found to exist in two different α and β crystalline forms. These forms can be obtained by:
(i) The α form of glucose is obtained by crystallisation from a concentrated solution of glucose at 303 K.
(ii) The β form of glucose is obtained by crystallisation from a concentrated solution of glucose at 303 K.
(iii) The β form is obtained by crystallisation from hot and saturated aqueous solution at 371 K.
(iv) The α form is obtained by crystallisation from hot and saturated aqueous solution at 371 K.
A solution of D-glucose in water rotates the plane polarised light ____________.
The α-D glucose and β-D glucose differ from each other due to difference in carbon atom with respect to its ____________.
Write the reactions of D-glucose which can’t be explained by its open-chain structure. How can cyclic structure of glucose explain these reactions?
On the basis of which evidences D-glucose was assigned the following structure?
\[\begin{array}{cc}
\ce{CHO}\\
|\phantom{....}\\
\phantom{..}\ce{(CHOH)4}\\
|\phantom{....}\\
\phantom{..}\ce{CH2OH}
\end{array}\]
Account for the following:
There are 5 OH groups in glucose
Consider the following reactions:
(i) \[\ce{Glucose + R-OH ->[Conc. HNO3] [A] ->[X eq of][(CH3CO)2O] Acetyl derivative}\]
(ii) \[\ce{Glucose ->[Ni/H2] [A] ->[Y eq of][(CH3CO)2O] Acetyl derivative}\]
(iii) \[\ce{Glucose ->[Z eq of][(CH3CO)2O] Acetyl derivative}\]
'X, 'Y' and 'Z' in these reactions are respectively:
