Advertisements
Advertisements
Question
Write a chemical equation for the following reaction:
Propanone is treated with dilute Ba( OH)2.
Solution
This is a self-aldol condensation reaction.
\[\begin{array}{cc}
\phantom{............}\ce{CH3} \phantom{........................}\ce{CH3}\phantom{}\\
\phantom{..........................}|\phantom{............................}|\phantom{.................}\\
\ce{\underset{\text{Acetone or propanone}}{2CH3COCH3}<-->[Dil. Ba(OH)2] CH3-C-CH2-CO-CH3->[ ][\Delta]CH3-\underset{\text{4-methyl pent-3-en-2-one}}{C=CH-CO-CH3 }+ H2O}\\
\phantom{..................................}|\phantom{........................} \text{(Aldol condensation product)}\phantom{..}\\
\ce{\underset{\text{4-Hydroxy-4-methyl pentan-2-one(ketol)}}{OH}}\phantom{...................}\\
\end{array}\]
RELATED QUESTIONS
A compound 'A' of molecular formula C2H3OCl undergoes a series of reactions as shown below. Write the structures of A, B, C and D in the following reactions :
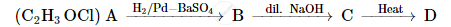
Write the products formed when CH3CHO reacts with the following reagents: CH3CHO in the presence of dilute NaOH
How will you convert ethanal into the following compound?
Butane-1, 3-diol
Complete the synthesis by giving missing starting material, reagent or product.
\[\begin{array}{cc}
\ce{C6H5CHO}\phantom{............}\\
\phantom{........}\ce{+\phantom{......}\ce{->[dil.NaOH][\Delta]}}\phantom{...}\\
\ce{CH3CH2CHO}\phantom{............}
\end{array}\]
Why is alpha (α) hydrogen of carbonyl compounds acidic in nature?
Explain aldol condensation reaction in detail.
Assertion: The α-hydrogen atom in carbonyl compounds is less acidic.
Reason: The anion formed after the loss of α-hydrogen atom is resonance stabilised.
Identify A and B from the following reaction:
\[\begin{array}{cc}
\ce{CH3}\phantom{.................}\\
|\phantom{....................}\\
\phantom{}\ce{2CH3 - C = O ->[Ba(OH)2] A ->[Δ] B + H2O}
\end{array}\]
\[\ce{CH3-CH2-CHO ->[dil][alkali] Product}\]
The product in the above reaction is:
Why is the α-hydrogens of aldehydes and ketones are acidic in nature?
