Science (English Medium)
Academic Year: 2018-2019
Date & Time: 13th March 2019, 10:30 am
Duration: 3h
Advertisements
- All questions are compulsory
- Section A: Q. no. 1 to 5 are very short-answer questions and carry 1 mark each.
- Section B: Q. no. 6 to 12 are short-answer questions and carry 2 marks each.
- Section C: Q. no. 13 to 24 are also short-answer questions and carry 3 marks each.
- Section D: Q. no. 25 to 27 are long-answer questions and carry 5 marks each.
Arrange the following in decreasing order of solubility in water:
\[\ce{(CH3)3N,(CH3)2NH,CH3NH2}\]
Chapter: [0.01] Solutions
Answer the following question.
What type of stoichiometric defect is shown by ZnS and why?
Chapter: [0.01] Solid State
Answer the following question.
Write one stereochemical difference between SN1 and SN2 reactions.
Chapter: [0.06] Haloalkanes and Haloarenes
Answer the following question.
Why are medicines more effective in the colloidal state?
Chapter: [0.05] Surface Chemistry
Answer the following question.
What is the difference between an emulsion and a gel?
Chapter: [0.05] Surface Chemistry
Answer the following question.
What is the basic structural difference between glucose and fructose?
Chapter: [0.1] Biomolecules
Answer the following question.
Write the products obtained after the hydrolysis of lactose.
Chapter: [0.1] Biomolecules
Answer the following question.
When MnO2 is fused with KOH in the presence of KNO3 as an oxidizing agent, it gives a dark green compound (A). Compound (A) disproportionates in an acidic solution to give a purple compound (B). An alkaline solution of compound (B) oxidizes KI to compound (C) whereas an acidified solution of compound (B) oxidizes KI to (D). Identify (A), (B), (C), and (D).
Chapter: [0.04] d-block and f-block Elements
Write the two applications of Henry's law.
Chapter: [0.01] Solutions
Answer the following question.
Write IUPAC name of the complex [Pt(en)2CI2]. Draw structures of geometrical isomers for this complex.
Chapter: [0.05] Coordination Compounds
Using IUPAC norms write the formulae for the following:
Hexaamminecobalt(III) sulphate
Chapter: [0.06] Haloalkanes and Haloarenes
Using IUPAC norms write the formulae for the following:
Potassium trioxalatochromate (III)
Chapter: [0.06] Haloalkanes and Haloarenes
Write a balanced chemical equation for the following process:
XeF2 undergoes hydrolysis.
Chapter:
Write a balanced chemical equation for the following process:
MnO2 is heated with conc.HCI
Chapter:
Arrange the following in the order of the property indicated against set :
H2O, H2S, H2Se, H2Te − increasing acidic character.
Chapter: [0.07] P - Block Elements
Arrange the following in order of the property indicated set.
HF, HCl, HBr, HI - decreasing bond enthalpy.
Chapter: [0.07] P - Block Elements
For a reaction
\[\ce{2H2O2->[I^-][Alkaline medium]2H2O + O2}\]
The Proposed mechanism is given below:
(1) H2O2+I- → H2O+IO-(slow)
(2) H2O2+IO-→H2O+I-+O2(fast)
(i) Write the rate law for the reaction.
(ii) Write the overall order of a reaction.
(iii) Out of steps(1) and (2), which one is the rate-determining step?
Chapter: [0.03] Chemical Kinetics
Write the hybridization and magnetic character of the following complexes:
[Fe(H2O)6]2+
(Atomic no. of Fe = 26)
Chapter: [0.05] Coordination Compounds
Write the hybridization and magnetic character of the following complexes:
[Fe(CO)5]
(Atomic no. of Fe = 26)
Chapter: [0.05] Coordination Compounds
Advertisements
Write the structure of main compounds A and B in the following reaction:
\[\ce{CH3CH2CN->[CH3MgBRH/3O+]A->[LiAIH4]B}\]
Chapter: [0.07] Alcohols, Phenols and Ethers
Write the structure of main compounds A and B in the following reaction: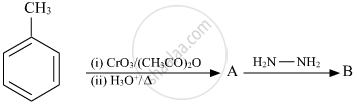
Chapter: [0.06] Haloalkanes and Haloarenes
Complete the following reaction:

Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Complete the following reaction:
\[\ce{(C6H5CH2)2Cd + 2CH3COCI}\]
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Complete the following reaction:
\[\begin{array}{cc}
\ce{CH3}\phantom{..............}\\
|\phantom{..............}\phantom{...}\\
\ce{CH3-CH-COOH->[(i) Br2/Red P4][(ii) H2O]}\\
\phantom{.......}\
\end{array}\]
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write a chemical equation for the following reaction:
Propanone is treated with dilute Ba( OH)2.
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write chemical equation of the following reaction :
Acetophenone is treated with `("Zn"("Hg"))/"Conc.HCl"`.
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write chemical equations of the following reaction :
Benzoyl chloride is hydrogenated in the presence of `"Pd"/(BaSO_4)`
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
What is the role of activated charcoal in gas masks?
Chapter: [0.01] Solutions
A colloidal sol is prepared by the given method in the figure. What is the charge on hydrated ferric oxide colloidal particles formed in the test tube? How is the sol represented?
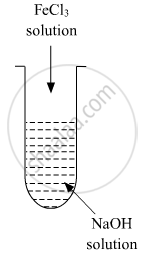
Chapter: [0.05] Surface Chemistry
How does chemisorption vary with temperature?
Chapter: [0.05] Surface Chemistry
A 4% solution(w/w) of sucrose (M = 342 g mol−1) in water has a freezing point of 271.15 K. Calculate the freezing point of 5% glucose (M = 180 g mol−1) in water.
(Given: Freezing point of pure water = 273.15 K)
Chapter: [0.01] Solutions
An element crystallizes in fcc lattice with a cell edge of 300 pm. The density of the element is 10.8 g cm−3. Calculate the number of atoms in 108 g of the element.
Chapter: [0.01] Solid State
How will you convert the following:
Impure Nickel to pure Nickel
Chapter:
How will you convert the following:
Zinc blende to Zinc metal
Chapter: [0.06] General Principles and Processes of Isolation of Elements
How will you convert the following:
[Ag(CN)2]− to Ag
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Give reasons for the following:
The transition metals generally form coloured compounds.
Chapter: [0.04] d-block and f-block Elements
Give reasons for the following:
E⁰ value for (Mn3+|Mn2+) is highly positive than that for (Cr3+|Cr2+) couple.
Chapter: [0.07] P - Block Elements
Give reasons for the following:
The chemistry of actinoids elements is not so smooth as that of the lanthanoids.
Chapter: [0.04] d-block and f-block Elements
Why type of drug is used in sleeping pills?
Chapter: [0.16] Chemistry in Everyday Life
What type of detergent are used in toothpastes?
Chapter: [0.16] Chemistry in Everyday Life
Why the use of alitame as an artificial sweetener is not recommended?
Chapter: [0.16] Chemistry in Everyday Life
Define the following term with a suitable example in each:
Broad-spectrum antibiotics
Chapter: [0.16] Chemistry in Everyday Life
Define the following term with a suitable example in each:
Disinfectants
Chapter: [0.16] Chemistry in Everyday Life
Define the following term with a suitable example in each:
Cationic detergents
Chapter: [0.16] Chemistry in Everyday Life
Write the structures of monomers used the following polymers:
Nylon - 6, 6
Chapter: [0.15] Polymers
Write the structures of monomers used the following polymers:
Glyptal
Chapter: [0.15] Polymers
Write the structures of monomers used the following polymers:
Buna S
Chapter: [0.15] Polymers
Is 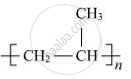 a homopolymer or copolymer? Give reason.
a homopolymer or copolymer? Give reason.
Chapter: [0.15] Polymers
Write the monomers of the following polymer :
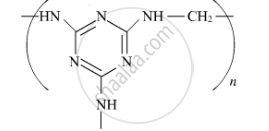
Chapter: [0.15] Polymers
What is the role of Sulphur in the vulcanization of rubber?
Chapter: [0.15] Polymers
Out of (CH3)3 C-Br and (CH3)3 C-I, which one is more reactive towards SN1 and why?
Chapter: [0.06] Haloalkanes and Haloarenes
Write the product formed when p-nitro chlorobenzene is heated with aqueous NaOH at 443K followed by acidification?
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Why dextro and laevo rotatory isomers of Butan-2-ol are difficult to separate by fractional distillation?
Chapter: [0.05] Coordination Compounds
Write equations of the following reactions:
Acetylation of aniline
Chapter: [0.09] Amines
Write equations of the following reactions:
Coupling reaction
Chapter: [0.09] Amines
Write equations of the following reactions:
Carbyl amine reaction
Chapter: [0.09] Amines
The decomposition of NH3 on a platinum surface is a zero-order reaction. If the rate constant (k) is 4 x 10-3 ms-1, how long will it take to reduce the initial concentration of NH3 from 0.1 M to 0.064 M?
Chapter: [0.03] Chemical Kinetics
Advertisements
Define the following term:
Oligosaccharides
Chapter: [0.1] Biomolecules
Define the following term:
Denaturation of protein
Chapter: [0.1] Biomolecules
Define the following term:
Vitamins
Chapter: [0.1] Biomolecules
Write the reactions involved when D-glucose is treated with the following reagent:
Br2 water
Chapter: [0.1] Biomolecules
Write the reactions involved when D-glucose is treated with the following reagent:
H2N-OH
Chapter: [0.1] Biomolecules
Write the reactions involved when D-glucose is treated with the following reagent:
(CH3CO)2O
Chapter: [0.1] Biomolecules
How do you convert the following :
Phenol to anisole
Chapter: [0.07] Alcohols, Phenols and Ethers
How do you convert the following :
Ethanol to Propan-2-ol
Chapter: [0.07] Alcohols, Phenols and Ethers
Write the mechanism of the following reaction :
\[\ce{C2H5OH->[H2SO4][443K]CH2=CH2 + H2O}\]
Chapter: [0.07] Alcohols, Phenols and Ethers
Why phenol undergoes electrophilic substitution more easily than benzene?
Chapter: [0.07] Alcohols, Phenols and Ethers
Account for the following:
o-nitrophenol is more steam volatile than p-nitrophenol.
Chapter: [0.07] Alcohols, Phenols and Ethers
Account for the following :
t-butyl chloride on heating with sodium methoxide gives 2-methylpropene instead of t-butyl methyl ether.
Chapter: [0.07] Alcohols, Phenols and Ethers
Write the equation involved in the following reaction:
Reimer-Tiemann reaction
Chapter: [0.07] Alcohols, Phenols and Ethers
Write the reaction involved in the following:
Friedal-Crafts Alkylation of Phenol
Chapter: [0.07] Alcohols, Phenols and Ethers
Give simple chemical test to distinguish between Ethanol and Phenol.
Chapter: [0.07] Alcohols, Phenols and Ethers
Give reasons for the following:
Sulphur in vapour state shows paramagnetic behaviour.
Chapter: [0.07] P - Block Elements
Give reasons for the following:
N-N bond is weaker than P-P bond.
Chapter: [0.05] Coordination Compounds
Give reasons for the following:
Ozone is thermodynamically less stable than oxygen.
Chapter: [0.07] P - Block Elements
Write the name of gas released when Cu is added to
dilute HNO3
Chapter: [0.07] P - Block Elements
Write the name of gas released when Cu is added to
conc. HNO3
Chapter: [0.07] P - Block Elements
Answer the following question.
Write the disproportionation reaction of H3PO3.
Chapter: [0.07] P - Block Elements
Draw the structure of XeF4.
Chapter: [0.07] P - Block Elements
Account for the following :
Although Fluorine has less negative electron gain enthalpy yet F2 is a strong oxidizing agent.
Chapter: [0.07] P - Block Elements
Account for the following :
Acidic character decreases from N2O3 to Bi2O3 in group 15.
Chapter: [0.07] P - Block Elements
Answer the following question.
Write a chemical reaction to test sulphur dioxide gas. write a chemical equation involved.
Chapter: [0.07] P - Block Elements
E°cell for the given redox reaction is 2.71 V
Mg(s) + Cu2+ (0.01 M) → Mg2+ (0.001 M) + Cu(s)
Calculate Ecell for the reaction. Write the direction of flow of current when an external opposite potential applied is
(i) less than 2.71 V and
(ii) greater than 2.71 V
Chapter: [0.02] Electrochemistry
Solve the following question.
A steady current of 2 amperes was passed through two electrolytic cells X and Y connected in series containing electrolytes FeSO4 and ZnSO4 until 2.8 g of Fe deposited at the cathode of cell X. How long did the current flow? Calculate the mass of Zn deposited at the cathode of cell Y.
(Molar mass : Fe = 56 g mol–1, Zn = 65.3 g mol–1, 1F = 96500 C mol–1)
Chapter: [0.02] Electrochemistry
In the plot of molar conductivity (∧m) vs square root of concentration (c1/2), following curves are obtained for two electrolytes A and B:
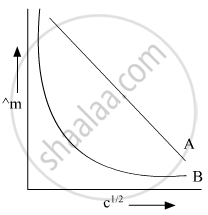
Answer the following:
(i) Predict the nature of electrolytes A and B.
(ii) What happens on extrapolation of ∧m to concentration approaching zero for electrolytes A and B?
Chapter: [0.02] Electrochemistry
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 12 Chemistry with solutions 2018 - 2019
Previous year Question paper for CBSE Class 12 -2019 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 12.
How CBSE Class 12 Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
