Advertisements
Advertisements
Question
Write the main products when
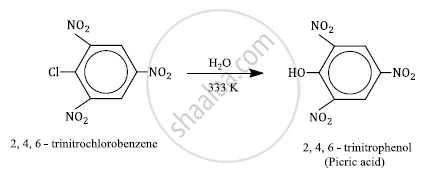
2, 4, 6-trinitrochlorobenzene is subjected to hydrolysis
Solution
2, 4, 6-trinitrochlorobenzene under mild hydrolysis conditions (H2O/323 K) gives 2, 4, 6-trinitrophenol or picric acid.
APPEARS IN
RELATED QUESTIONS
Write the final product(s) in each of the following reactions:
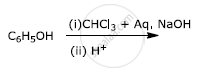
Write the equation involved in the following reaction:
Kolbe’s reaction
Name the reagent used in the following reaction:
Bromination of phenol to 2, 4, 6-tribromophenol.
Picric acid is ____________.
When phenol is treated with excess bromine water, it gives:
\[\ce{C2H5OH + SOCl2 ->[Pyridine] C2H5Cl + SO2 + HCl}\]
The above reaction is known as:
Which of the following compounds is aromatic alcohol?
| (A) |  |
| (B) | 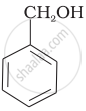 |
| (C) | 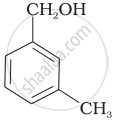 |
| (D) | 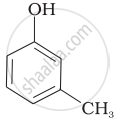 |
Convert the following:
Phenol to N-phenylethanamide.
Write the chemical equation involved in the following reactions:
Acetylation of salicylic add
For the pair phenol and cyclohexanol, answer the following:
Give one chemical test to distinguish between the two.
