Advertisements
Advertisements
Questions
Write the molecular formula and structure of cyclohexane. How many covalent bonds are there in a molecule of cyclohexane?
Draw the structure of the molecule of cyclohexane (C6H12)·
Solution
The molecular formula of cyclohexane is C6H12.
Its structure is as follows:
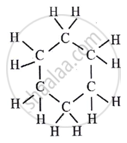
A cyclohexane molecule has six carbon-carbon single bonds and twelve carbon-hydrogen single bonds. Therefore, there are 18 covalent bonds in one molecule of cyclohexane.
Notes
Students should refer to the answer according to their questions.
APPEARS IN
RELATED QUESTIONS
Draw the structures for Butanone
The pair of elements which exhibits the property of catenation is:
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
Which compound contains single bonds as well as a double bond?
You are given the following molecular formulae of some hydrocarbons:
Which formula represents benzene?
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which three formulae can represent cyclic hydrocarbons?
Which of the following are correct structural isomers of butane?
- \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H - C - C - C - C - H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}
\end{array}\] - \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\\
\ce{H - C - C - C - H}\\
|\phantom{.....}\backslash\phantom{..}|\\
\phantom{.....}\ce{H}\phantom{.......}\ce{C - H}\\
\phantom{.........}|\\
\phantom{.........}\ce{H}
\end{array}\] - \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\\
\ce{H - C - C - C - H}\\
|\phantom{....}\phantom{....}|\\
\ce{H}\phantom{........}\ce{H}\\
|\\
\ce{H - C - H}\\
|\\\ce{H}
\end{array}\] - \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\\
\ce{H - C - C - H}\\
|\phantom{....}|\\
\ce{H - C - C - H}\\
|\phantom{....}|\\
\ce{H}\phantom{...}\ce{H}
\end{array}\]
Catenation is the ability of an atom to form bonds with other atoms of the same element. It is exhibited by both carbon and silicon. Compare the ability of catenation of the two elements. Give reasons.
How will you prove that C4H8 and C5H10 are homologues?
Recognise the carbon chain type of the following:
\[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\\\ce{H - C - C - C - C - H}\\|\phantom{....}|\phantom{....}|\phantom{....}|\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}
\end{array}\]
Draw two structural isomers of butane.
