Advertisements
Advertisements
Question
Write the name of the cell which is generally used in transistors. Write the reactions taking place at the anode and the cathode of this cell.
Solution
The dry cell (also known as Leclanche cell) is used in transistors.
The reactions taking place at the anode and cathode are given below.
At anode: Zn(s) → Zn2+ + 2e−
At cathode : `MnO_2 + NH_4^+ + e^(-) -> MnO(OH) + NH_3`
APPEARS IN
RELATED QUESTIONS
96500 coulombs correspond to the charge on how many electrons?
Following reactions occur at cathode during the electrolysis of aqueous sodium chloride solution:
Na+(aq) + e− ⟶ Na (s) E0 = 2.71 V
H+(aq) + e− ⟶ `1/2` H2 (g) E0 = 0.00 V
On the basis of their standard reduction electrode potential (E0) values, which reaction is feasible at the cathode and why?
A solution of \[\ce{Ni(NO3)2}\] is electrolysed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of \[\ce{Ni}\] is deposited at the cathode?
On passing 1.5 F charge, the number of moles of aluminium deposited at cathode are _______ [Molar mass of Al = 27 gram mol–1]
(A) 1.0
(B) 13.5
(C) 0.50
(D) 0.75
Draw neat labelled diagram of electrolytic refining of blister copper
Following reactions occur at cathode during the electrolysis of aqueous copper(II) chloride solution :
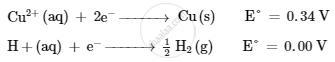
On the basis of their standard reduction electrode potential (E°) values, which reaction is feasible at the cathode and why ?
How much quantity of electricity in coulomb is required to deposit 1.346 × 10-3 kg of Ag in 3.5 minutes from AgNO3 solution?
( Given: Molar mass of Ag is 108 × 10-3 kg mol-1 )
Electrolytic cell uses electrical energy to bring about ____________.
The quantity of electricity needed to separately electrolyse 1 M solution of ZnSO4, AlCl3, and AgNO3 completely is in the ratio of ______.
On passing electricity through nitrobenzene solution, it is converted into azobenzene. The mass of azobenzene is ______ mg, if the same quantity of electricity produces oxygen just sufficient to burn 96 mg of fullerene (C60)·
