SSC (English Medium)
SSC (Marathi Semi-English)
Academic Year: 2023-2024
Date & Time: 18th March 2024, 11:00 am
Duration: 2h
Advertisements
Note -
- All questions are compulsory.
- Use of a calculator is not allowed.
- The numbers to the right of the questions indicate full marks.
- In case of MCQs [Q. No. 1(A)] only the first attempt will be evaluated and will be given credit.
- Scientifically correct, labelled diagrams should be drawn wherever necessary.
The SI unit of heat energy is ______.
joule
calorie
kilo calorie
Cal/g °C
none of these
Kcal/kg
Chapter: [0.05] Heat
We can see the sun even when it is little below the horizon because of ______.
Reflection of light
Refraction of light
Dispersion of light
Absorption of light
Chapter: [0.06] Refraction of Light [0.17] Wonders of Light 2
______ is the functional group of carboxylic acid.
\[\ce{-COOH}\]
\[\ce{-CO-}\]
\[\ce{-CHO-}\]
\[\ce{-OH}\]
Chapter: [0.09] Carbon Compounds
In simple microscope ______ lens is used.
Concave
Plano concave
Plano convex
Convex
Chapter: [0.07] Lenses
In ______ process a layer of molten tin is deposited on metals.
Anodization
Tinning
Galvanizing
Alloying
Chapter: [0.03] Chemical Reactions and Equations [0.08] Metallurgy
Write the name and symbol of the element from the description.
The atom having the smallest size.
Chapter: [0.02] Periodic Classification of Elements
Write the molecular formula of calcium carbonate.
Chapter: [0.03] Chemical Reactions and Equations [0.12] The Magic of Chemical Reactions
Identify the hydrocarbon from the given electron-dot structure:
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{....}\ce{H}\phantom{}\\
\phantom{}\ce{H}\phantom{..}\ce{\text{}^\bullet_\bullet}\phantom{..}\ce{\overset{\bullet\phantom{.}\bullet}{\underset{\bullet\phantom{.}\bullet}{C}}}\phantom{..}\ce{\text{}^\bullet_\bullet}\phantom{..}\ce{\overset{\bullet\phantom{.}\bullet}{\underset{\bullet\phantom{.}\bullet}{C}}}\phantom{..}\ce{\text{}^\bullet_\bullet}\phantom{..}\ce{H}\phantom{}\\
\phantom{}\ce{H}\phantom{....}\ce{H}\phantom{}\\
\end{array}\]
Chapter: [0.09] Carbon Compounds
Match the Columns:
| Column ‘A’ | Column ‘B’ |
| Refractive index of water | (a) 1.31 |
| (b) 1.36 | |
| (c) 1.33 |
Chapter: [0.06] Refraction of Light [0.17] Wonders of Light 2
Advertisements
Give scientific reason.
When the gas formed on heating limestone, is passed through freshly prepared lime water, the lime water turns milky.
Chapter: [0.03] Chemical Reactions and Equations [0.12] The Magic of Chemical Reactions
Give a scientific reason.
Tungsten metal is used to make a solenoid type coil in an electric bulb.
Chapter: [0.04] Effects of Electric Current [0.14] The Electric Spark
Write scientific reason.
On exposure to air, silver articles turn blackish after some time.
Chapter: [0.03] Chemical Reactions and Equations [0.08] Metallurgy
Identify the figure and explain their use.

Chapter: [0.04] Effects of Electric Current
What is meant by a satellite launch vehicle?
Chapter: [0.1] Space Missions
Name any one Indian satellite launch vehicle.
Chapter: [0.1] Space Missions
If focal length of a convex lens is 20 cm at what is the power of the lens?
Chapter: [0.07] Lenses [0.16] Wonders of Light 1
Advertisements
Select the appropriate options and complete the following paragraph:
(metals, non-metals, metalloids, four, seven, s-block, p-block, d-block, f-block).
On the basis of electronic configuration, elements in the modern periodic table are classified Into ______ blocks. Groups 1 and 2 elements are included in ______ and all these elements are metals. (except Hydrogen). Group 13 to 18 elements are included in ______. This block contains metals, non-metals and metalloids. Group 3 to 12 elements are included in ______ and all the elements are ______ elements shown at the bottom of the periodic table i.e. Lanthanides and Actinides constitute ______ and all these elements are metals.
Chapter: [0.02] Periodic Classification of Elements [0.11] School of Elements
What are the factors affecting the rate of chemical reaction?
Chapter: [0.03] Chemical Reactions and Equations
Explain any one factor affecting the rate of chemical reaction.
Chapter: [0.03] Chemical Reactions and Equations
Observe the following graph and answer the following questions:

- What does the graph represent?
- What does the line AB represent?
- What does the line BC represent?
Chapter: [0.05] Heat
Complete the following table by observing the given figures:
| Figure → | 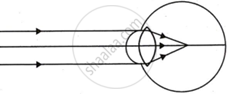 |
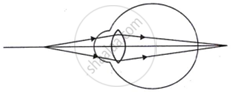 |
| Points ↓ | ||
| (a) Name of the defect | ______ | ______ |
| (b) Position of the image | ______ | ______ |
| (c) Lens used to correct the defect | ______ | ______ |
Chapter: [0.07] Lenses [0.16] Wonders of Light 1
Observe the figure and answer the questions:
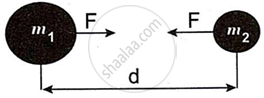
- State Newton's universal law of gravitation.
- If the distance between the two bodies is tripled, how will the gravitational force between them change?
- What will happen to gravitational force, if mass of one of the object is doubled?
Chapter: [0.01] Gravitation
The orbit of a satellite is exactly 35780 km above the earth's surface and its tangential velocity is 3.08 km/s.
How much time the satellite will take to complete one revolution around the earth?
(Radius of earth = 6400 km.)
Chapter: [0.1] Space Missions
What is a solenoid?
Chapter: [0.04] Effects of Electric Current [0.15] All about Electromagnetism
Draw a neat diagram of a solenoid and name its various components.
Chapter: [0.04] Effects of Electric Current [0.15] All about Electromagnetism
Observe the given diagram and answer the questions:
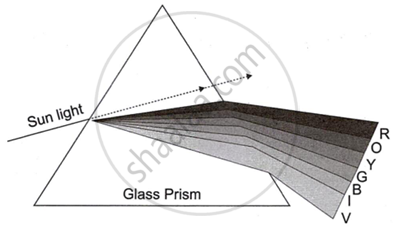
- Name the process shown in the figure.
- Name the colour that deviates the most.
- Name the colour that deviates the least.
- Name any one phenomenon in nature which is based on the above process.
- Define ‘spectrum’.
Chapter: [0.06] Refraction of Light
Observe the diagram given below and answer the questions:
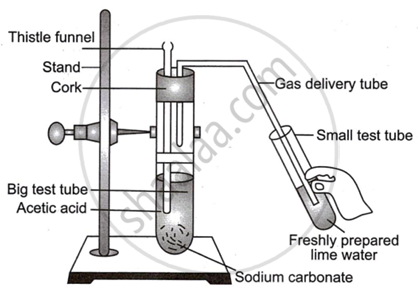
- Name the reactants in this reaction.
- Which gas comes out as effervescence in the bigger test tube?
- What is the colour change in the lime water?
- In the above experiment instead of sodium carbonate which chemical can be used to get same products?
- Write the use of acetic acid.
Chapter: [0.09] Carbon Compounds
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
Maharashtra State Board previous year question papers 10th Standard Board Exam Science and Technology 1 with solutions 2023 - 2024
Previous year Question paper for Maharashtra State Board 10th Standard Board Exam -2024 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Science and Technology 1, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of Maharashtra State Board 10th Standard Board Exam.
How Maharashtra State Board 10th Standard Board Exam Question Paper solutions Help Students ?
• Question paper solutions for Science and Technology 1 will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
