Advertisements
Advertisements
प्रश्न
Consider the cyclic process ABCA, shown in figure, performed on a sample of 2.0 mol of an ideal gas. A total of 1200 J of heat is withdrawn from the sample in the process. Find the work done by the gas during the part BC.
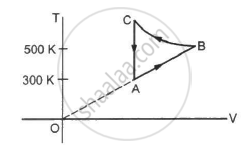
उत्तर
Given:-
Number of moles of the gas, n = 2
∆Q = − 1200 J (Negative sign shows that heat is extracted out from the system)
∆U = 0 ..............(During cyclic process)
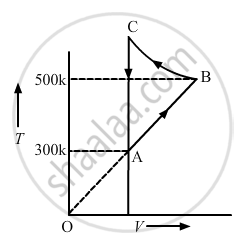
Using the first law of thermodynamics, we get
∆Q = ∆U + ∆W
⇒ −1200 = 0 + (WAB + WBC + WCA)
Since the change in volume of the system applies on line CA, work done during CA will be zero.
From the ideal gas equation, we get
PV = nRT
P∆V = nR∆T
W = P∆V = nR∆T
⇒ ∆Q = ∆U + ∆W
⇒ −1200 = nR∆T + WBC + 0
⇒ −1200 = 2 × 8.3 × 200 + WBC
WBC = − 400 × 8.3 − 1200
= − 4520 J
APPEARS IN
संबंधित प्रश्न
Write the mathematical expression of the First Law of Thermodynamics for the Isobaric process.
The first law of thermodynamics is a statement of ____________ .
Answer the following in one or two sentences.
State the first law of thermodynamics.
A resistor held in running water carries electric current. Treat the resistor as the system
- Does heat flow into the resistor?
- Is there a flow of heat into the water?
- Is any work done?
- Assuming the state of resistance to remain unchanged, apply the first law of thermodynamics to this process.
For an Isochoric process
A sample of gas absorbs 4000 kJ of heat and surrounding does 2000 J of work on sample. What is the value of ∆U?
The compressibility of water is 5 × 10-10 m2/N. Pressure of 15 × 106 Pa is applied on 100 ml volume of water. The change in the volume of water is ______.
Based on first law of thermodynamics which of the following is correct.
In a given process for an ideal gas, dW = 0 and dQ < 0. Then for the gas ____________.
If an average person jogs, hse produces 14.5 × 103 cal/min. This is removed by the evaporation of sweat. The amount of sweat evaporated per minute (assuming 1 kg requires 580 × 103 cal for evaparation) is ______.
Consider two containers A and B containing identical gases at the same pressure, volume and temperature. The gas in container A is compressed to half of its original volume isothermally while the gas in container B is compressed to half of its original value adiabatically. The ratio of final pressure of gas in B to that of gas in A is ______.
The first law of thermodynamics is concerned with the conservation of ______.
An electric appliance supplies 6000 J/min heat to the system. If the system delivers a power of 90 W. How long it would take to increase the internal energy by 2.5 × 103 J?
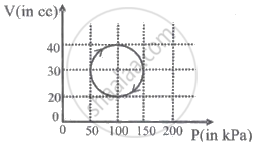
A system is taken through a cyclic process represented by a circle as shown. The heat absorbed by the system is ______.
The amount of work done in increasing the voltage across the plates of capacitor from 5 V to 10 V is W. The work done in increasing it from 10 V to 15 V will be ______.
104 J of work is done on a certain volume of a gas. If the gas releases 125 kJ of heat, calculate the change in internal energy of the gas.
What is Isobaric process?
A monoatomic gas at 27°C is adiabatically compressed to 80% of its initial volume. Find the final temperature of the gas.
What is an isothermal process?
