Advertisements
Advertisements
प्रश्न
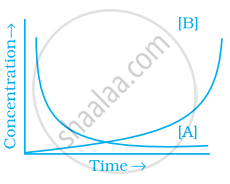
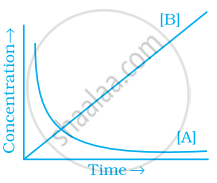
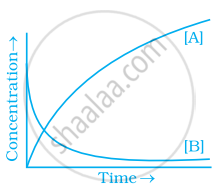
Consider the reaction A ⇌ B. The concentration of both the reactants and the products varies exponentially with time. Which of the following figures correctly describes the change in concentration of reactants and products with time?
विकल्प
उत्तर
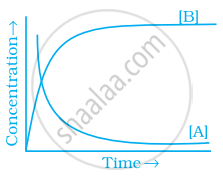
Explanation:
If \[\ce{A -> B}\] then the concentration of both reactants and the products very exponentially with time. But graph the reactant concentration decreases exponentially and the product concentration increases.
APPEARS IN
संबंधित प्रश्न
From the rate expression for the following reaction, determine the order of reaction and the dimension of the rate constant.
\[\ce{CH3CHO_{(g)} -> CH4_{(g)} + CO_{(g)}}\] Rate = k [CH3CHO]3/2
A reaction is first order in A and second order in B. Write the differential rate equation.
A reaction is first order in A and second order in B. How is the rate affected on increasing the concentration of B three times?
Write resonating structures of ozone.
Rate of reaction for the combustion of propane is equal to:
\[\ce{C3H8_{(g)} + 5O2_{(g)} -> 3CO2_{(g)} + 4H2O_{(g)}}\]
Why can we not determine the order of a reaction by taking into consideration the balanced chemical equation?
Assertion: Rate constants determined from Arrhenius equation are fairly accurate for simple as well as complex molecules.
Reason: Reactant molecules undergo chemical change irrespective of their orientation during collision.
For a first order A → B, the reaction rate at reactant concentration of 0.01 m is found to be 2.0 × 10–5. The half-life period of reaction.
For a chemical reaction starting with some initial concentration of reactant At as a function of time (t) is given by the equation,
`1/("A"_"t"^4) = 2 + 1.5 xx 10^-3` t
The rate of disappearance of [A] is ____ × 10-2 M/sec when [A] = 2 M.
[Given: [At] in M and t in sec.]
[Express your answer in terms of 10-2 M /s]
[Round off your answer if required]
Which of the following statement is true?