Advertisements
Advertisements
प्रश्न
Explain the isobaric process and derive the work done in this process.
उत्तर
Isobaric process: This is a thermodynamic process that occurs at constant pressure. Even though the pressure is constant in this process, temperature, volume and internal energy are not constant. From the ideal gas equation, we have
V = `((μ"R")/"P")"T"` .........(1)
Here, `(μ"R")/"P"` = constant
In an isobaric process the temperature is directly proportional to volume.
V ∝ T (Isobaric process) …........(2)
This implies that for an isobaric process, the V-T graph is a straight line passing through the origin.
If gas goes from a state (Vi, Ti) to (Vf, Tf) at constant pressure, then the system satisfies the following equation
`"T"_"f"/"V"_"f" = "T"_"i"/"V"_"i"` ...........(3)
Examples for Isobaric process:
(i) When the gas is heated and pushes the piston so that it exerts a force equivalent to atmospheric pressure plus the force due to gravity then this process is isobaric.
(ii) Most of the cooking processes in our kitchen are isobaric processes. When the food is cooked in an open vessel, the pressure above the food is always at atmospheric pressure. The PV diagram for an isobaric process is a horizontal line parallel to the volume axis. Figure (a) represents the isobaric process where volume decreases figure
(b) represents the isobaric process where volume increases.

PV diagram for an isobaric process
The work done in an isobaric process: Work done by the gas
W = `int_("V"_"i")^("V"_"f") "PdV"` ..........(4)
In an isobaric process, the pressure is constant, so P comes out of the integral,
W = `"P" int_("V"_"i")^("V"_"f") "dV"` ........(5)
W = P[Vf – Vi] = PΔV ........(6)
Where ∆V denotes the change in the volume. If ∆V is negative, W is also negative. This implies that the work is done on the gas. If ∆V is positive, W is also positive, implying that work is done by the gas equation.
The equation (6) can also be rewritten using the ideal gas equation.
From ideal gas equation
PV = μRT and V = `(μ"RT")/"P"`
Substituting this in equation (6) we get
W = `μ"RT"_"f" (1 - "T"_"i"/"T"_"f")` ...........(7)
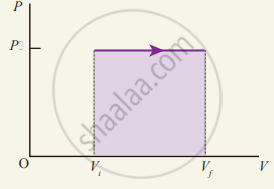
Work done in an isobaric process
In the PV diagram, the area under the isobaric curve is equal to the work done in the isobaric process. The shaded area in the above diagram is equal to the work done by the gas.
The first law of thermodynamics for the isobaric process is given by
ΔU = Q − PΔV ......(8)
W = PΔV, ΔU = `"Q" - μ"RT"_"f" [1 - "T"_"i"/"T"_"f"]`
APPEARS IN
संबंधित प्रश्न
Differentiate between the reversible and irreversible processes.
An ideal gas of volume 2 L is adiabatically compressed to (1/10)th of its initial volume. Its initial pressure is 1.01 x 105 Pa, calculate the final pressure. (Given 𝛾 = 1.4)
Explain thermodynamics of the adiabatic process.
Apply first law for an isobaric process.
Can the given heat energy be completely converted to work in a cyclic process? If not, when can the heat can completely converted to work?
What are the limitations of the first law of thermodynamics?
In an adiabatic expansion of the air, the volume is increased by 4%, what is the percentage change in pressure? (For air γ = 1.4)
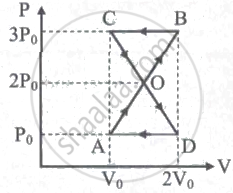
A thermodynamic system undergoes cyclic process ABCDA as shown in the figure. The work done by the system is ______
In which of the following processes, beat is neither absorbed nor released by a system?
Give any two types of a thermodynamic process.
