Advertisements
Advertisements
प्रश्न
The average kinetic energy of molecules in a gas at temperature T is 1.5 kT. Find the temperature at which the average kinetic energy of the molecules of hydrogen equals the binding energy of its atoms. Will hydrogen remain in molecular from at this temperature? Take k = 8.62 × 10−5 eV K−1.
उत्तर
Average kinetic energy (K) of the molecules in a gas at temperature (T) is given by
K = `3/2 kT`
Here,
k = 8.62 × 10−5 eVK−1
T = Temperature of gas
The binding energy of hydrogen atom is 13.6 eV.
According to the question,
Average kinetic energy of hydrogen molecules = Binding energy of hydrogen atom
∴ 1.5 kT = 13.6
⇒ 1.5 × 8.62 × 10−5 × T = 13.6
`rArr T = (13.6)/(1.5xx8.62xx10^-5)`
No, it is impossible for hydrogen to remain in molecular state at such a high temperature.
APPEARS IN
संबंधित प्रश्न
Classically, an electron can be in any orbit around the nucleus of an atom. Then what determines the typical atomic size? Why is an atom not, say, a thousand times bigger than its typical size? The question had greatly puzzled Bohr before he arrived at his famous model of the atom that you have learnt in the text. To simulate what he might well have done before his discovery, let us play as follows with the basic constants of nature and see if we can get a quantity with the dimensions of length that is roughly equal to the known size of an atom (~ 10−10 m).
(a) Construct a quantity with the dimensions of length from the fundamental constants e, me, and c. Determine its numerical value.
(b) You will find that the length obtained in (a) is many orders of magnitude smaller than the atomic dimensions. Further, it involves c. But energies of atoms are mostly in non-relativistic domain where c is not expected to play any role. This is what may have suggested Bohr to discard c and look for ‘something else’ to get the right atomic size. Now, the Planck’s constant h had already made its appearance elsewhere. Bohr’s great insight lay in recognising that h, me, and e will yield the right atomic size. Construct a quantity with the dimension of length from h, me, and e and confirm that its numerical value has indeed the correct order of magnitude.
When white radiation is passed through a sample of hydrogen gas at room temperature, absorption lines are observed in Lyman series only. Explain.
What will be the energy corresponding to the first excited state of a hydrogen atom if the potential energy of the atom is taken to be 10 eV when the electron is widely separated from the proton? Can we still write En = E1/n2, or rn = a0 n2?
In which of the following systems will the radius of the first orbit (n = 1) be minimum?
Which of the following curves may represent the speed of the electron in a hydrogen atom as a function of trincipal quantum number n?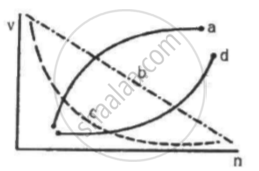
As one considers orbits with higher values of n in a hydrogen atom, the electric potential energy of the atom
An electron with kinetic energy 5 eV is incident on a hydrogen atom in its ground state. The collision
Which of the following products in a hydrogen atom are independent of the principal quantum number n? The symbols have their usual meanings.
(a) vn
(b) Er
(c) En
(d) vr
Find the radius and energy of a He+ ion in the states (a) n = 1, (b) n = 4 and (c) n = 10.
(a) Find the first excitation potential of He+ ion. (b) Find the ionization potential of Li++ion.
Find the maximum Coulomb force that can act on the electron due to the nucleus in a hydrogen atom.
A hydrogen atom in a state having a binding energy of 0.85 eV makes transition to a state with excitation energy 10.2 e.V (a) Identify the quantum numbers n of the upper and the lower energy states involved in the transition. (b) Find the wavelength of the emitted radiation.
Find the maximum angular speed of the electron of a hydrogen atom in a stationary orbit.
Find the temperature at which the average thermal kinetic energy is equal to the energy needed to take a hydrogen atom from its ground state to n = 3 state. Hydrogen can now emit red light of wavelength 653.1 nm. Because of Maxwellian distribution of speeds, a hydrogen sample emits red light at temperatures much lower than that obtained from this problem. Assume that hydrogen molecules dissociate into atoms.
In a hydrogen atom the electron moves in an orbit of radius 0.5 A° making 10 revolutions per second, the magnetic moment associated with the orbital motion of the electron will be ______.
Let En = `(-1)/(8ε_0^2) (me^4)/(n^2h^2)` be the energy of the nth level of H-atom. If all the H-atoms are in the ground state and radiation of frequency (E2 - E1)/h falls on it ______.
- it will not be absorbed at all.
- some of atoms will move to the first excited state.
- all atoms will be excited to the n = 2 state.
- no atoms will make a transition to the n = 3 state.
Positronium is just like a H-atom with the proton replaced by the positively charged anti-particle of the electron (called the positron which is as massive as the electron). What would be the ground state energy of positronium?
A hydrogen atom makes a transition from n = 5 to n = 1 orbit. The wavelength of photon emitted is λ. The wavelength of photon emitted when it makes a transition from n = 5 to n = 2 orbit is ______.
