Advertisements
Advertisements
प्रश्न
The conversion of molecules X to Y follows second order kinetics. If concentration of X is increased to three times how will it affect the rate of formation of Y?
उत्तर
The reaction \[\ce{X -> Y}\] follows second order kinetics.
Therefore, the rate equation for this reaction will be:
Rate = k[X]2
If the concentration of X is increased to three times, then [r'] = k[3X]2
`("r'")/"r" = ("k"[3"X"]^2)/("k"["X"]^2)` = 9
Hence, the rate of formation will increase by 9 times.
APPEARS IN
संबंधित प्रश्न
For a reaction: 
Rate = k
(i) Write the order and molecularity of this reaction.
(ii) Write the unit of k.
For a reaction A + B ⟶ P, the rate is given by
Rate = k [A] [B]2
How is the rate of reaction affected if the concentration of B is doubled?
For a chemical reaction R → P, the variation in the concentration (R) vs. time (t) plot is given as:
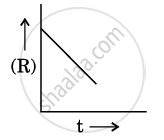
(i) Predict the order of the reaction.
(ii) What is the slope of the curve ?
(iii) Write the unit of rate constant for this reaction.
For the reaction: \[\ce{2A + B → A2B}\] the rate = k[A][B]2 with k = 2.0 × 10−6 mol−2 L2 s−1. Calculate the initial rate of the reaction when [A] = 0.1 mol L−1, [B] = 0.2 mol L−1. Calculate the rate of reaction after [A] is reduced to 0.06 mol L−1.
For a reaction R ---> P, half-life (t1/2) is observed to be independent of the initial concentration of reactants. What is the order of reaction?
Write the principle behind the following methods of refining:
Hydraulic washing
Which of the following statement is true for order of a reaction?
Consider a first order gas phase decomposition reaction given below :
\[\ce{A(g) -> B(g) + C(g)}\]
The initial pressure of the system before decomposition of A was pi. After lapse of time ‘t’, total pressure of the system increased by x units and became ‘pt’ The rate constant k for the reaction is given as ______.
Rate law for the reaction \[\ce{A + 2B -> C}\] is found to be Rate = k [A][B]. Concentration of reactant ‘B’ is doubled, keeping the concentration of ‘A’ constant, the value of rate constant will be ______.
Consider the reaction A ⇌ B. The concentration of both the reactants and the products varies exponentially with time. Which of the following figures correctly describes the change in concentration of reactants and products with time?
In any unimolecular reaction:
(i) only one reacting species is involved in the rate determining step.
(ii) the order and the molecularity of slowest step are equal to one.
(iii) the molecularity of the reaction is one and order is zero.
(iv) both molecularity and order of the reaction are one.
Why is the probability of reaction with molecularity higher than three very rare?
Why does the rate of any reaction generally decreases during the course of the reaction?
Assertion: Rate constants determined from Arrhenius equation are fairly accurate for simple as well as complex molecules.
Reason: Reactant molecules undergo chemical change irrespective of their orientation during collision.
The role of a catalyst is to change
In the presence of a catalyst, the heat evolved or absorbed during the reaction.
The rate constant for the reaction \[\ce{2H2O5 -> 4NO2 + O2}\] is 30 × 10–5 sec–1. if the rate is 204 × 10–5 mol L–1 S–1, then the concentration of N2O5 (in mol–1) is-
For a first order A → B, the reaction rate at reactant concentration of 0.01 m is found to be 2.0 × 10–5. The half-life period of reaction.
The conversion of molecules A to B follow second order kinetics. If concentration of A is increased to three times, how will it affect the rate of formation of B?
A reaction is second order with respect to a reactant. How is the rate of reaction affected if the concentration of the reactant is reduced to half?
