Advertisements
Advertisements
प्रश्न
Match the atomic number with the following:
A metal of valency one.
पर्याय
19
15
8
4
2
उत्तर
19
APPEARS IN
संबंधित प्रश्न
Give the trend in metallic character:
down the group top to bottom.
iii) Observe the figure and answer the following questions.
a) Identify the block shown by box A and write an electronic configuration of any one element of this block.
b) Identify the block of element denoted by letter B and write its period number.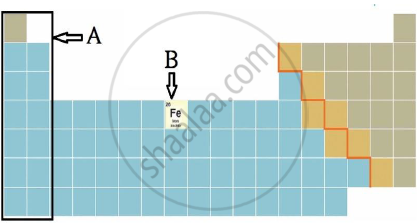
In the third period, which is the most metallic and most non-metallic element?
Electropositivity means _______.
Silicon is a metallic element.
On moving down the group metallic character increases.
Write scientific reason.
The metallic character of elements increases while going down the groups.
Write scientific reason.
The non-metallic character increases while going from left to right in a period.
3, 1, 2 electrons are in valence shells of X, Y, Z elements. From this information, state the group in which they belong and write their valencies.
Metals are good ______ because they are electron ______.
