Advertisements
Advertisements
Question
A gas is enclosed in a cylindrical vessel fitted with a frictionless piston. The gas is slowly heated for some time. During the process, 10 J of heat is supplied and the piston is found to move out 10 cm. Find the increase in the internal energy of the gas. The area of cross section of the cylinder = 4 cm2 and the atmospheric pressure = 100 kPa.
Solution
Given:- Heat supplied to the system, ∆Q = 10 J
Change in volume of the system, ∆V = Area of cross section × Displacement of the piston
= A × 10 cm
= (4 × 10) cm3 = 40 × 10−6 m3
P = 100 kPa
∆W = P∆V = 100 × 103 × 40 × 10−6 m3
= 4 J
∆U = ?
Using the first law of thermodynamics, we get
10 = ∆U + ∆W
⇒ 10 = ∆U + 4
⇒ ∆U = 6 J
Here, positive sign indicates that the internal energy of the system has increased.
APPEARS IN
RELATED QUESTIONS
An electric heater supplies heat to a system at a rate of 100W. If the system performs work at a rate of 75 Joules per second. At what rate is the internal energy increasing?
The first law of thermodynamics is a statement of ____________ .
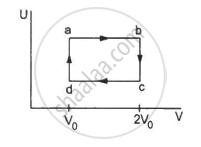
Figure shows the variation in the internal energy U with the volume V of 2.0 mol of an ideal gas in a cyclic process abcda. The temperatures of the gas at b and c are 500 K and 300 K respectively. Calculate the heat absorbed by the gas during the process.
A solar cooker and a pressure cooker both are used to cook food. Treating them as thermodynamic systems, discuss the similarities and differences between them.
Answer the following in one or two sentences.
State the first law of thermodynamics.
ΔU is equal to ____________ work.
A sample of gas absorbs 4000 kJ of heat and surrounding does 2000 J of work on sample, what is the value of ΔU?
Three copper blocks of masses M1, M2 and M3 kg respectively are brought into thermal contact till they reach equilibrium. Before contact, they were at T1, T2, T3 (T1 > T2 > T3). Assuming there is no heat loss to the surroundings, the equilibrium temprature T is (s is specific heat of copper)
Consider a cycle tyre being filled with air by a pump. Let V be the volume of the tyre (fixed) and at each stroke of the pump ∆V(V) of air is transferred to the tube adiabatically. What is the work done when the pressure in the tube is increased from P1 to P2?
The initial state of a certain gas is (Pi, Vi, Ti). It undergoes expansion till its volume becomes Vf. Consider the following two cases:
- the expansion takes place at constant temperature.
- the expansion takes place at constant pressure.
Plot the P-V diagram for each case. In which of the two cases, is the work done by the gas more?
A cycle followed by an engine (made of one mole of an ideal gas in a cylinder with a piston) is shown in figure. Find heat exchanged by the engine, with the surroundings for each section of the cycle. (Cv = (3/2)R)
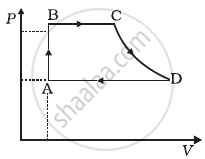
- AB : constant volume
- BC : constant pressure
- CD : adiabatic
- DA : constant pressure
Write the mathematical equation for the first law of thermodynamics for:
Adiabatic process
Mathematical equation of first law of thermodynamics for isochoric process is ______.
One mole of an ideal gas is allowed to expand reversibly and adiabatically from a temperature of 27°C. If the work done during the process is 3 kJ, the final temperature will be equal to ______.
(Cv = 20 JK−1)
Which among the following equations represents the first law of thermodynamics under isobaric conditions?
A soap bubble in vacuum has a radius of 3 cm and another soap bubble in vacuum has a radius of 4 cm. If the two bubbles coalesce under isothermal condition, then the radius of the new bubble is ______.
Derive an expression for the work done during an isothermal process.
In an adiabatic process, ______.
Show that the heat absorbed at constant pressure is equal to the change in enthalpy of the system.
