Advertisements
Advertisements
Question
The internal energy of a gas is given by U = 1.5 pV. It expands from 100 cm3 to 200 cm3against a constant pressure of 1.0 × 105 Pa. Calculate the heat absorbed by the gas in the process.
Solution
Let change in volume of the gas be ∆V.
∆V = (200 − 100) cm3 = 100 cm3
= 10−4 m3
p = 1 × 105 Pa
Change in internal energy of the system, ∆U = 1.5 pV
∆U = 1.5 × 105 × 10−4 = 15 J
∆W = p∆V
= 105 × 10−4 = 10 J
Using the first law of thermodynamics, we get
∆Q = ∆U + ∆W = 10 + 15 = 25 J
Thus, heat absorbed by the system is 25 J.
APPEARS IN
RELATED QUESTIONS
When we heat an object, it expands. Is work done by the object in this process? Is heat given to the object equal to the increase in its internal energy?
Calculate the change in internal energy of a gas kept in a rigid container when 100 J of heat is supplied to it.
Find the change in the internal energy of 2 kg of water as it is heated from 0°C to 4°C. The specific heat capacity of water is 4200 J kg−1 K−1 and its densities at 0°C and 4°C are 999.9 kg m−3 and 1000 kg m−3 respectively. Atmospheric pressure = 105 Pa.
A resistor held in running water carries electric current. Treat the resistor as the system
- Does heat flow into the resistor?
- Is there a flow of heat into the water?
- Is any work done?
- Assuming the state of resistance to remain unchanged, apply the first law of thermodynamics to this process.
For an Isochoric process
10 kg of four different gases (Cl2, CH4, O2, N2) expand isothermally and reversibly from 20 atm to 10 atm. The order of amount of work will be ____________.
Two moles of an ideal gas is expanded isothermally and reversibly at 300 K from 1 L to 10 L. The enthalpy change in kJ is ______.
Which of the following are TRUE for a reversible isothermal process?
(i) ∆U = 0
(ii) ∆H = 0
(iii) Q = W
(iv) ∆T = 0
The isothermal bulk modulus of a perfect gas at pressure P is numerically equal to ____________.
A sample of gas absorbs 4000 kJ of heat and surrounding does 2000 J of work on sample, what is the value of ΔU?
An ideal gas undergoes cyclic process ABCDA as shown in given P-V diagram (figure). The amount of work done by the gas is ______.

An ideal gas undergoes isothermal process from some initial state i to final state f. Choose the correct alternatives.
- dU = 0
- dQ= 0
- dQ = dU
- dQ = dW
The initial state of a certain gas is (Pi, Vi, Ti). It undergoes expansion till its volume becomes Vf. Consider the following two cases:
- the expansion takes place at constant temperature.
- the expansion takes place at constant pressure.
Plot the P-V diagram for each case. In which of the two cases, is the work done by the gas more?
Consider one mole of perfect gas in a cylinder of unit cross section with a piston attached (figure). A spring (spring constant k) is attached (unstretched length L) to the piston and to the bottom of the cylinder. Initially the spring is unstretched and the gas is in equilibrium. A certain amount of heat Q is supplied to the gas causing an increase of volume from V0 to V1.
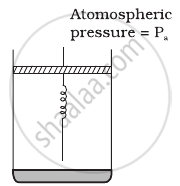
- What is the initial pressure of the system?
- What is the final pressure of the system?
- Using the first law of thermodynamics, write down a relation between Q, Pa, V, Vo and k.
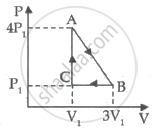
An ideal gas is taken through series of changes ABCA. The amount of work involved in the cycle is ______.
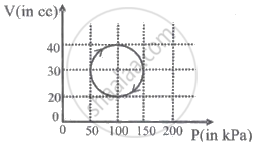
A system is taken through a cyclic process represented by a circle as shown. The heat absorbed by the system is ______.
The V cc volume of gas having `γ = 5/2` is suddenly compressed to `(V/4)` cc. The initial pressure of the gas is p. The final pressure of the gas will be ______.
An ideal gas having pressure p, volume V and temperature T undergoes a thermodynamic process in which dW = 0 and dQ < 0. Then, for the gas ______.
What is true for an adiabatic process?
