Advertisements
Advertisements
Question
An ideal gas undergoes cyclic process ABCDA as shown in given P-V diagram (figure). The amount of work done by the gas is ______.

Options
6P0V0
–2P0V0
+ 2P0V0
+ 4P0V0
Solution
An ideal gas undergoes cyclic process ABCDA as shown in given P-V diagram (figure). The amount of work done by the gas is `underline(- 2P_0V_0)`.
Explanation:
Consider the P-V diagram given in the question.
Work done in the process ABCD = Area of rectangle ABCDA
= (AB) × BC
= (3V0 – V0) × (2P0 – P0)
= 2V0 × P0
= 2p0V0
As the process is going anticlockwise, hence there is a net compression in the gas. So, work done by the gas = –2P0V0.
APPEARS IN
RELATED QUESTIONS
Write the mathematical expression of the First Law of Thermodynamics for Isothermal Process
A system can be taken from the initial state p1, V1 to the final state p2, V2 by two different methods. Let ∆Q and ∆W represent the heat given to the system and the work done by the system. Which of the following must be the same in both the methods?
The internal energy of a gas is given by U = 1.5 pV. It expands from 100 cm3 to 200 cm3against a constant pressure of 1.0 × 105 Pa. Calculate the heat absorbed by the gas in the process.
Find the change in the internal energy of 2 kg of water as it is heated from 0°C to 4°C. The specific heat capacity of water is 4200 J kg−1 K−1 and its densities at 0°C and 4°C are 999.9 kg m−3 and 1000 kg m−3 respectively. Atmospheric pressure = 105 Pa.
Calculate the increase in the internal energy of 10 g of water when it is heated from 0°C to 100°C and converted into steam at 100 kPa. The density of steam = 0.6 kg m−3. Specific heat capacity of water = 4200 J kg−1 °C−1 and the latent heat of vaporization of water = 2.25 × 10 6J kg−1.
Answer the following in one or two sentences.
State the first law of thermodynamics.
Change in internal energy, when 4 KJ of work is done on the system and 1 KJ heat is given out by the system, is:
A cycle followed by an engine (made of one mole of perfect gas in a cylinder with a piston) is shown in figure.
A to B : volume constant
B to C : adiabatic
C to D : volume constant
D to A : adiabatic
VC = VD = 2VA = 2VB
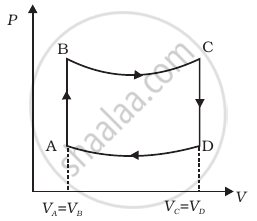
- In which part of the cycle heat is supplied to the engine from outside?
- In which part of the cycle heat is being given to the surrounding by the engine?
- What is the work done by the engine in one cycle? Write your answer in term of PA, PB, VA.
- What is the efficiency of the engine?
(γ = `5/3` for the gas), (Cv = `3/2` R for one mole)
An electric appliance supplies 6000 J/min heat to the system. If the system delivers a power of 90 W. How long it would take to increase the internal energy by 2.5 × 103 J?
One mole of an ideal gas is initially kept in a cylinder with a movable frictionless and massless piston at pressure of 1.01MPa, and temperature 27°C. It is then expanded till its volume is doubled. How much work is done if the expansion is isobaric?
