Advertisements
Advertisements
Question
Calculate the increase in the internal energy of 10 g of water when it is heated from 0°C to 100°C and converted into steam at 100 kPa. The density of steam = 0.6 kg m−3. Specific heat capacity of water = 4200 J kg−1 °C−1 and the latent heat of vaporization of water = 2.25 × 10 6J kg−1.
Solution
Given:-
Mass of water, m = 10 g = 0.01 kg
Pressure, P = 105 Pa
Specific heat capacity of water, c = 1000 J/Kg °C
Latent heat, L = 2.25 × 106 J/Kg
∆ t = Change in temperature of the system = 100°C = 373 K
∆Q = Heat absorbed to raise the temperature of water from 0°C to 100°C + Latent heat for conversion of water to steam
∆Q = \[mc ∆ t + mL\]
= 0.01 × 4200 × 100 + 0.01 × 2.5 × 106
= 4200 + 25000 = 29200 J
∆W = P∆V
∆V \[= \text{mass}\left( \frac{1}{\text{final density}} - \frac{1}{\text{initial density}} \right)\]
\[∆ V = \left( \frac{0 . 01}{0 . 6} \right) - \left( \frac{0 . 01}{1000} \right) = 0 . 01699\]
∆W = P∆V = 0.01699 × 105 = 1699 J
Using the first law, we get
∆Q = ∆W + ∆U
∆U = ∆Q − ∆W = 29200 − 1699
= 27501 = 2.75 × 104 J
APPEARS IN
RELATED QUESTIONS
When we heat an object, it expands. Is work done by the object in this process? Is heat given to the object equal to the increase in its internal energy?
A system can be taken from the initial state p1, V1 to the final state p2, V2 by two different methods. Let ∆Q and ∆W represent the heat given to the system and the work done by the system. Which of the following must be the same in both the methods?
50 cal of heat should be supplied to take a system from the state A to the state B through the path ACB as shown in figure. Find the quantity of heat to be suppled to take it from A to B via ADB.

A solar cooker and a pressure cooker both are used to cook food. Treating them as thermodynamic systems, discuss the similarities and differences between them.
A sample of gas absorbs 4000 kJ of heat and surrounding does 2000 J of work on sample. What is the value of ∆U?
The process, in which no heat enters or leaves the system, is termed as ____________.
The isothermal bulk modulus of a perfect gas at pressure P is numerically equal to ____________.
The initial state of a certain gas is (Pi, Vi, Ti). It undergoes expansion till its volume becomes Vf. Consider the following two cases:
- the expansion takes place at constant temperature.
- the expansion takes place at constant pressure.
Plot the P-V diagram for each case. In which of the two cases, is the work done by the gas more?
A cycle followed by an engine (made of one mole of perfect gas in a cylinder with a piston) is shown in figure.
A to B : volume constant
B to C : adiabatic
C to D : volume constant
D to A : adiabatic
VC = VD = 2VA = 2VB
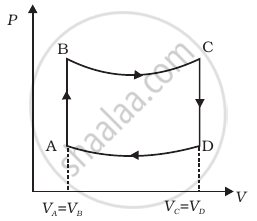
- In which part of the cycle heat is supplied to the engine from outside?
- In which part of the cycle heat is being given to the surrounding by the engine?
- What is the work done by the engine in one cycle? Write your answer in term of PA, PB, VA.
- What is the efficiency of the engine?
(γ = `5/3` for the gas), (Cv = `3/2` R for one mole)
A cycle followed by an engine (made of one mole of an ideal gas in a cylinder with a piston) is shown in figure. Find heat exchanged by the engine, with the surroundings for each section of the cycle. (Cv = (3/2)R)
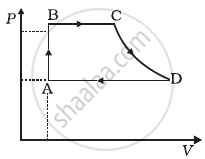
- AB : constant volume
- BC : constant pressure
- CD : adiabatic
- DA : constant pressure
Write the mathematical equation for the first law of thermodynamics for:
Adiabatic process
A system is taken through a cyclic process represented by a circle as shown. The heat absorbed by the system is ______.
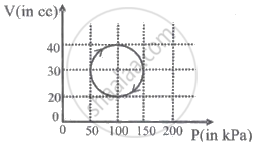
What work will be done, when 3 moles of an ideal gas are compressed to half the initial volume at a constant temperature of 300 K?
An ideal gas (γ = 1.5) is expanded adiabatically. How many times has the gas had to be expanded to reduce the root mean square velocity of molecules two times?
An ideal gas having pressure p, volume V and temperature T undergoes a thermodynamic process in which dW = 0 and dQ < 0. Then, for the gas ______.
Using the first law of thermodynamics, show that for an ideal gas, the difference between the molar specific heat capacities at constant pressure and at constant volume is equal to the molar gas constant R.
A monoatomic gas at 27°C is adiabatically compressed to 80% of its initial volume. Find the final temperature of the gas.
Calculate work done when 2 moles of ideal gas expands by 5 dm3 isothermally at pressure 1.2 bar.
Write a short note on isobar.
