Advertisements
Advertisements
Question
- Draw the structures of all isomeric alcohols of molecular formula C5H12O and give their IUPAC names.
- Classify the isomers of alcohols in the above question as primary, secondary and tertiary alcohols.
Solution
i. The isomeric alcohols with molecular formula C5H12O are –
(a) \[\ce{\underset{Pentanol (1^\circ)}{CH3 - CH2 - CH2 - CH2 - CH2 - OH}}\]
(b) \[\begin{array}{cc}
\ce{CH3 - CH2 - CH2 - \overset{∗}{C}H - CH3}\\
\phantom{............}|\\
\phantom{...............}\ce{\underset{Pentan-2-ol (2^\circ)}{OH}}\
\end{array}\]
(c) \[\begin{array}{cc}
\ce{CH3 - CH2 - CH - CH2 - CH3}\\
|\phantom{..}\\
\phantom{..}\ce{\underset{Pentan-3-ol (2^\circ)}{OH}}\
\end{array}\]
(d) \[\begin{array}{cc}
\ce{H3C - H2C - H\overset{∗}{C} - CH2OH}\\
\phantom{.....}|\\
\phantom{.........}\ce{\underset{2-Methylbutan-1-ol (1^\circ)}{CH3}}\
\end{array}\]
(e) \[\begin{array}{cc}
\ce{CH3}\phantom{............}\\
|\phantom{...............}\\
\ce{\underset{3-Methylbutan-1-ol (1^\circ)}{CH3 - CH - CH2 - CH2 - OH}}\\
\end{array}\]
(f) \[\begin{array}{cc}
\ce{CH3}\phantom{....}\\
|\phantom{.......}\\
\ce{CH3 - C - CH2 - CH3}\\
|\phantom{.......}\\
\ce{\underset{2-Methylbutan-2-ol (3^\circ)}{OH}}\phantom{....}\
\end{array}\]
(g) \[\begin{array}{cc}
\ce{CH3}\phantom{...}\\
|\phantom{......}\\
\ce{CH3 - C - CH2 - OH}\\
|\phantom{......}\\
\ce{\underset{2, 2-Dimethylpropan-1-ol (1^\circ)}{CH3}}\phantom{..}\
\end{array}\]
(h) \[\begin{array}{cc}
\phantom{.}\ce{CH3}\phantom{...}\ce{OH}\\
|\phantom{......}|\phantom{..}\\
\ce{\underset{3-Methylbutan-2-ol (2^\circ)}{CH3 - CH - \underset{∗}{C}H - CH3}}\
\end{array}\]
Isomers (b), (d) and (h) contain chiral carbon atoms; thus, they exhibit enantiomerism.
ii. Isomers (a), (d), (e) and (g) are primary alcohols.
Isomers (b), (c) and (h) are secondary alcohols.
Isomer (f) is a tertiary alcohol.
APPEARS IN
RELATED QUESTIONS
What is metamerism?
Write IUPAC name of the following compound:
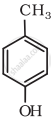
Write IUPAC name of the following compound:
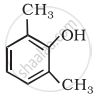
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - O - CH2 - CH - CH3}\\
\phantom{..........}|\\
\phantom{............}\ce{CH3}
\end{array}\]
How is phenol converted into the following?
benzene
Write the IUPAC name of the following :
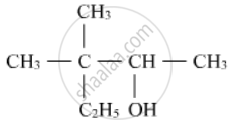
What.will be the product fonned when chlorobenzene is heated with sodium metal in the presence of dry ether?
Write structural formulae for 1-Ethylcyclohexanol.
Write IUPAC name of the following
\[\begin{array}{cc}\ce{CH3-CH-CH-CH2-OH}\\|\phantom{.....}|\phantom{.......}\\\ce{OH}\phantom{..}\ce{CH3}\phantom{.....}\end{array}\]
In a carbinol system of nomenclature tert.butyl alcohol is named as _______________
Give IUPAC names of the following compound:
\[\begin{array}{cc}
\phantom{..}\ce{H}\phantom{...}\ce{CH3}\phantom{.}\ce{H}\phantom{..}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{}\\
\ce{H - C - C - C - H}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{}\\
\phantom{.}\ce{H}\phantom{...}\ce{OH}\phantom{.}\ce{H}\phantom{.}\\
\end{array}\]
HBr reacts fastest with ____________.
n-Propyl alcohol and isopropyl alcohol can be chemically distinguished by which reagent?
When ethyl alcohol reacts with acetic acid, the products formed are:
Which of the following gives a positive iodoform test?
Among the following sets of reactants which one produces anisole?
Which of the following reagents can be used to oxidise primary alcohols to aldehydes?
(i) \[\ce{CrO3}\] in anhydrous medium.
(ii) \[\ce{KMnO4}\] in acidic medium.
(iii) Pyridinium chlorochromate.
(iv) Heat in the presence of Cu at 573 K.
Assertion: Addition reaction of water to but-1-ene in acidic medium yields butan-1-ol.
Reason: Addition of water in acidic medium proceeds through the formation of primary carbocation.
