Advertisements
Advertisements
Question
Explain formation of peptide linkage in protein with an example.
Solution
Proteins are polymers of α-amino acid, and a huge number of α-amino acid peptide bonds connect them (amide bond).
A peptide bond is a chemical link produced between two molecules when one of their carboxyl groups combines with the amino group of the other releasing a water molecule (H2O). This is a dehydration synthesis reaction (also known as a condensation reaction) that happens most commonly between amino acids.
For example:

APPEARS IN
RELATED QUESTIONS
How are proteins classified on the basis of molecular shapes?
What is peptide linkage?
Define the following as related to proteins
Peptide linkage
What are the common types of secondary structure of proteins?
What type of bonding helps in stabilising the ∝-helix structure of proteins?
Write one difference between α-helix and β-pleated structures of proteins.
Differentiate between the following :
Peptide linkage and Glycosidic linkage
The protein responsible for blood clotting is ____________.
The correct statement for protein haemoglobin.
Which functional group participates in disulphide bond formation in proteins?
Proteins are found to have two different types of secondary structures viz. α-helix and β-pleated sheet structure. α-helix structure of protein is stabilised by:
Dinucleotide is obtained by joining two nucleotides together by phosphodiester linkage. Between which carbon atoms of pentose sugars of nucleotides are these linkages present?
Optical rotations of some compounds along with their structures are given below which of them have D configuration.
| (I) | 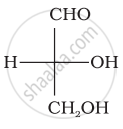 |
| (II) | 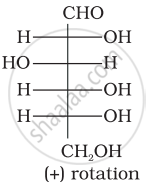 |
| (III) | 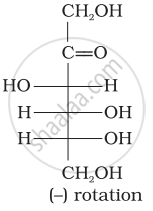 |
In fibrous proteins, polypeptide chains are held together by:
(i) van der Waals forces
(ii) disulphide linkage
(iii) electrostatic forces of attraction
(iv) hydrogen bonds
α-Helix is a secondary structure of proteins formed by twisting of polypeptide chain into right-handed screw like structures. Which type of interactions are responsible for making the α-helix structure stable?
Protein found in a biological system with a unique three-dimensional structure and biological activity is called a native protein. When a protein in its native form, is subjected to a physical change like change in temperature or a chemical change like, change in pH, denaturation of protein takes place. Explain the cause.
Which moieties of nucleosides are involved in the formation of phosphodiester linkages present in dinucleotides? What does the word diester in the name of linkage indicate? Which acid is involved in the formation of this linkage?
Assertion: β-glycosidic linkage is present in maltose,

Reason: Maltose is composed of two glucose units in which C–1 of one glucose unit is linked to C–4 of another glucose unit.
Explain the terms primary and secondary structure of proteins. What is the difference between α-helix and β-pleated sheet structure of proteins?
The main structural feature of proteins is
Out of the following, which type of interaction is responsible for the stabilisation of the α-helix structure of proteins?
Assertion (A): Proteins are polymers of α-amino acids connected by a peptide bond.
Reason (R): A tetrapeptide contains 4 amino acids linked by 4 peptide bonds.
β-pleated sheet structure in proteins refers to ______.
Write a classification of proteins with an example.
