Advertisements
Advertisements
Question
Obtain the binding energy (in MeV) of a nitrogen nucleus `(""_7^14"N")`, given `"m"(""_7^14"N")` = 14.00307 u.
Solution
Atomic mass of nitrogen `(""_7^14"N")`, m = 14.00307 u
A nucleus of nitrogen `""_7^14"N"` contains 7 protons and 7 neutrons.
Hence, the mass defect of this nucleus, Δm = 7mH + 7mn − m
Where,
Mass of a proton, mH = 1.007825 u
Mass of a neutron, mn= 1.008665 u
∴ Δm = 7 × 1.007825 + 7 × 1.008665 − 14.00307
= 7.054775 + 7.06055 − 14.00307
= 0.11236 u
But 1 u = 931.5 MeV/c2
∴ Δm = 0.11236 × 931.5 MeV/c2
Hence, the binding energy of the nucleus is given as:
Eb = Δmc2
Where,
c = Speed of light
∴ Eb = `0.11236 xx 931.5(("MeV")/"c"^2) xx "c"^2`
= 104.66334 MeV
Hence, the binding energy of a nitrogen nucleus is 104.66334 MeV.
APPEARS IN
RELATED QUESTIONS
Derive an expression for the total energy of electron in ‘n' th Bohr orbit. Hence show that energy of the electron is inversely proportional to the square of principal quantum number. Also define binding energy.
Obtain the binding energy of the nuclei `""_26^56"Fe"` and `""_83^209"Bi"` in units of MeV from the following data:
`"m"(""_26^56"Fe")` = 55.934939 u
`"m"(""_83^209"Bi")`= 208.980388 u
What is meant by the terms half-life of a radioactive substance and binding energy of a nucleus?
Use this graph to explain the release of energy in both the processes of nuclear fusion and fission.
Is it easier to take out a nucleon (a) from carbon or from iron (b) from iron or from lead?
In which of the following decays the atomic number decreases?
(a) α-decay
(b) β+-decay
(c) β−-decay
(d) γ-decay
Binding energy per nucleon for helium nucleus (2 He) is 7.0 MeV Find value of mass defect for helium nucleus
Sketch a graph showing the variation of binding energy per nucleon of a nucleus with its mass number.
The figure shows the plot of binding energy (BE) per nucleon as a function of mass number A. The letters A, B, C, D, and E represent the positions of typical nuclei on the curve. Point out, giving reasons, the two processes (in terms of A, B, C, D, and E ), one of which can occur due to nuclear fission and the other due to nuclear fusion.
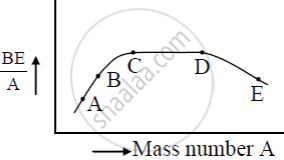
Answer the following question.
Draw the curve showing the variation of binding energy per nucleon with the mass number of nuclei. Using it explains the fusion of nuclei lying on the ascending part and fission of nuclei lying on the descending part of this curve.
The difference in mass of a nucleus and its constituents is called ______.
The deuteron is bound by nuclear forces just as H-atom is made up of p and e bound by electrostatic forces. If we consider the force between neutron and proton in deuteron as given in the form of a Coulomb potential but with an effective charge e′: F = `1/(4πε_0) e^('2)/r` estimate the value of (e’/e) given that the binding energy of a deuteron is 2.2 MeV.
Nuclei with magic no. of proton Z = 2, 8, 20, 28, 50, 52 and magic no. of neutrons N = 2, 8, 20, 28, 50, 82 and 126 are found to be very stable.
(i) Verify this by calculating the proton separation energy Sp for 120Sn (Z = 50) and 121Sb = (Z = 51).
The proton separation energy for a nuclide is the minimum energy required to separate the least tightly bound proton from a nucleus of that nuclide. It is given by `S_P = (M_(z-1^' N) + M_H - M_(ZN))c^2`.
Given 119In = 118.9058u, 120Sn = 119.902199u, 121Sb = 120.903824u, 1H = 1.0078252u.
(ii) What does the existance of magic number indicate?
Define binding energy per nucleon.
State the significance of binding energy per nucleon.
What is binding energy of nucleus?
Find the binding energy per nucleon of 235U based on the information given below.
| Mass(u) | |
| mass of neutral `""_92^235"U"` | 235.0439 |
| mass of a proton | 1.0073 |
| mass of a neutron | 1.0087 |
