Advertisements
Advertisements
Question
State Boyle's Law.
Solution 1
Robert Boyle systematically studied the relationship between pressure and volume of gases. In 1962, he found that, at a constant temperature, the volume of a fixed mass of a dry gas decreased by half when the pressure on it was doubled, and it became four times its original volume when its pressure was decreased to on-fourth. He described this behaviour in the form of a law, known as Boyle's Law.
Solution 2
Boyle’s Law : “Temperature remaining constant the volume of a given mass of dry gas is inversely proportional to its pressure.”
V α `1/"P"` = T = Constant
APPEARS IN
RELATED QUESTIONS
Explain Boyle's Law on the basis of the kinetic theory of matter.
State the law which is represented by the following graph:
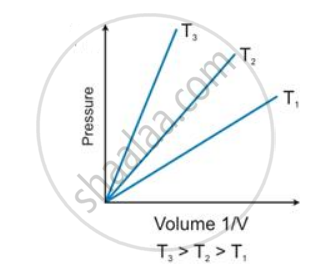
Give reasons for the following:
Inflating a balloon seems to violate Boyle's law.
A steel cylinder of internal volume 20 litres is filled with hydrogen at 29 atmospheric pressure. If hydrogen is used to fill a balloon at 1.25 atmospheric pressure at the same temperature, what volume will the gas occupy?
A certain amount of a gas occupies a volume of 0.4 litre at 17°C. To what temperature should it be heated so that its volume gets (a) doubled, (b) reduced to half, pressure remaining constant?
A certain mass of a gas occupies 2 litres at 27°C and 100 Pa. Find the temperature when volume and pressure become half of their initial values.
50 cm3 of hydrogen is collected over water at 17°C and 750 mmHg pressure. Calculate the volume of a dry gas at STP. The water vapour pressure at 17°C is 14 mmHg.
A given mass of a gas occupied 143 cm3 at 27° C and 700 mm Hg pressure. What will be its volume at 300 K and 280 mm Hg pressure?
Assuming temperature remaining constant calculate the pressure of the gas in the following:
The pressure of a gas having volume 380 lits. originally occupying 800 cm3 at 76 cm. pressure.
According to Boyle’s law, the shape of the graph between pressure and reciprocal of volume is _______.
