Advertisements
Advertisements
Question
Sucrose decomposes in acid solution into glucose and fructose according to the first order rate law with `"t"_(1/2)`= 3 hours. What fraction of the sample of sucrose remains after 8 hours?
Solution
`"t"_(1/2)` = 3 Hours
Now we know that,
k = `0.693/"t"_(1/2)`
= `0.693/3`
= 0.231 hr−1
Put above value in the formula of first order reaction,
k = `2.303/8log [R]_0/([R])`
So,
`log [R]_0/([R])=(0.231xx8)/2.303` = 0.8024
Taking antilog on both sides,
`[R]_0/([R])` = 6.3445
`[[R]]/[R]_0` = 0.158
Fraction of the sample of sucrose remaining after 8 hours = 0.158
APPEARS IN
RELATED QUESTIONS
A first order reaction has a rate constant 1.15 × 10−3 s−1. How long will 5 g of this reactant take to reduce to 3 g?
Time required to decompose SO2Cl2 to half of its initial amount is 60 minutes. If the decomposition is a first order reaction, calculate the rate constant of the reaction.
The following data were obtained during the first order thermal decomposition of SO2Cl2 at a constant volume.
\[\ce{SO2Cl2_{(g)} -> SO2_{(g)} + Cl2_{(g)}}\]
| Experiment | Time/s–1 | Total pressure/atm |
| 1 | 0 | 0.5 |
| 2 | 100 | 0.6 |
Calculate the rate of the reaction when total pressure is 0.65 atm.
In a pseudo first order hydrolysis of ester in water, the following results were obtained:
| t/s | 0 | 30 | 60 | 90 |
| [A]/mol L−1 | 0.55 | 0.31 | 0.17 | 0.085 |
Calculate the average rate of reaction between the time interval 30 to 60 seconds.
The rate constant for a first order reaction is 60 s−1. How much time will it take to reduce the initial concentration of the reactant to its `1/16`th value?
For the decomposition of azoisopropane to hexane and nitrogen at 543 K, the following data are obtained.
| t (sec) | P(mm of Hg) |
| 0 | 35.0 |
| 360 | 54.0 |
| 720 | 63.0 |
Calculate the rate constant.
The time required for 10% completion of a first order reaction at 298 K is equal to that required for its 25% completion at 308 K. If the value of A is 4 × 1010 s−1. Calculate k at 318 K and Ea.
Following data are obtained for reaction :
N2O5 → 2NO2 + 1/2O2
| t/s | 0 | 300 | 600 |
| [N2O5]/mol L–1 | 1.6 × 10-2 | 0.8 × 10–2 | 0.4 × 10–2 |
1) Show that it follows first order reaction.
2) Calculate the half-life.
(Given log 2 = 0.3010, log 4 = 0.6021)
A first order reaction takes 20 minutes for 25% decomposition. Calculate the time when 75% of the reaction will be completed.
(Given : log = 2 = 0·3010, log 3 = 0·4771, log 4 = 0·6021)
Show that the time required for 99.9% completion of a first-order reaction is three times the time required for 90% completion.
Which of the following graphs is correct for a first order reaction?

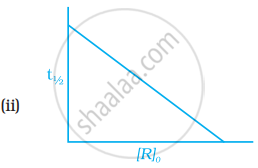
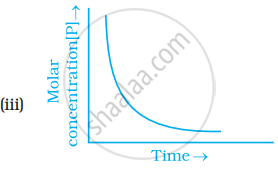
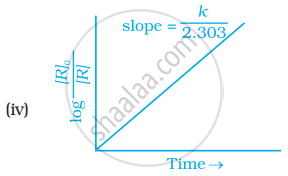
State a condition under which a bimolecular reaction is kinetically first order reaction.
First order reaction is 50% complete in 1.26 × 1014s. How much time could it take for 100% completion?
A first order reaction is 50% complete in 20 minute What is rate constant?
The reaction X → product
Follow first order of kinetics. In 40 minutes the concentration of 'X' changes from 0.1 m to 0.025. M. The rate of reaction when concentration of X is 0.01 m is.
Time required to decompose SO2Cl2 to half of its initial concentration is 60 minutes. If the de-composite is a first order reaction, calculated the rate constant of the reaction-
Gaseous cyclobutene isomerizes to butadiene in a first order process which has a 'k' value of 3.3 × 10−4 s−1 at 153°C. The time in minutes it takes for the isomerization to proceed 40% to completion at this temperature is ______. (Rounded-off to the nearest integer)
The decomposition of formic acid on gold surface follows first-order kinetics. If the rate constant at 300 K is 1.0 × 10−3 s−1 and the activation energy Ea = 11.488 kJ mol−1, the rate constant at 200 K is ______ × 10−5 s−1. (Round off to the Nearest Integer)
(Given R = 8.314 J mol−1 K−1)
The reaction \[\ce{SO2Cl2(g) -> SO2(g) + Cl2(g)}\] is a first-order gas reaction with k = 2.2 × 10−5 sec−1 at 320°C. The percentage of SO2Cl2 is decomposed on heating this gas for 90 min, is ______%.
For a first order reaction, the ratio of the time for 75% completion of a reaction to the time for 50% completion is ______. (Integer answer)
The slope in the plot of ln[R] vs. time for a first order reaction is ______.
The slope in the plot of `log ["R"]_0/(["R"])` Vs. time for a first-order reaction is ______.
How will you represent first order reactions graphically?
What is the rate constant?
The following data were obtained during the decomposition of SO2Cl2 at the constant volume. SO2Cl2 →SO2(g) + Cl2(g)
| Time (s) | Total Pressure (bar) |
| 0 | 0.5 |
| 100 | 0.6 |
Calculate the rate constant of the reaction.
Slove: \[\ce{2NOBr -> 2NO_{2(g)} + Br_{2(g)}}\]
For the above reaction, the rate law is rate = k[NOBr]2. If the rate of reaction is 6.5 × 10−6 mol L−1 s−1 at 2 × 10−3 mol L−1 concentration of NOBr, calculate the rate constant k for the reaction.
Write the equation for integrated rate law for a first order reaction.
Show that `t_(1/2)= 0.693/k` for first reaction.
Write the unit of rate constant [k] for the first order reaction.
