Advertisements
Advertisements
Question
The molar conductivity of CH3COOH at infinite dilution is 390 Scm2/mol. Using the graph and given information, the molar conductivity of CH3COOK will be:
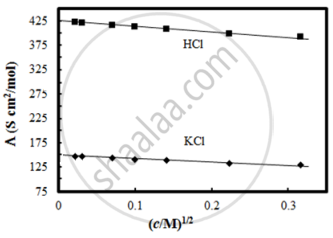
Options
100 Scm2/mol
115 Scm2/mol
150 Scm2/mol
125 Scm2/mol
Solution
115 Scm2/mol
Explanation:
Λ°CH3COOK = Λ°CH3COOH + Λ°KCl – Λ°HCl
= 390 + 150 – 425
= 115 Scm2/mol
APPEARS IN
RELATED QUESTIONS
State Kohlrausch Law
Why does the conductivity of a solution decrease with dilution?
Conductivity of 0.00241 M acetic acid is 7.896 × 10−5 S cm−1. Calculate its molar conductivity and if `∧_"m"^0` for acetic acid is 390.5 S cm2 mol−1, what is its dissociation constant?
Write mathematical expression of molar conductivity of the given solution at infinite dilution.
Molar conductivity denoted by the symbol Λm is related to the conductivity of the solution by the equation (k is the conductivity and c is the concentration).
\[\ce{Λ^0_m}_{(NH_4OH)}\] is equal to ______.
\[\ce{Λ^0_m H2O}\] is equal to:
(i) \[\ce{Λ^0_m_{(HCl)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaCl)}}}}\]
(ii) \[\ce{Λ^0_m_{(HNO_3)} + \ce{Λ^0_m_{(NaNO_3)} - \ce{Λ^0_m_{(NaOH)}}}}\]
(iii) \[\ce{Λ^0_{(HNO_3)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaNO_3)}}}}\]
(iv) \[\ce{Λ^0_m_{(NH_4OH)} + \ce{Λ^0_m_{(HCl)} - \ce{Λ^0_m_{(NH_4Cl)}}}}\]
Assertion: `"E"_("Ag"^+ //"Ag")` increases with increase in concentration of Ag+ ions.
Reason: `"E"_("Ag"^+ //"Ag")` has a positive value.
The variation of molar conductivity with concentration of an electrolyte (X) m aqueous solution is shown in the given figure.
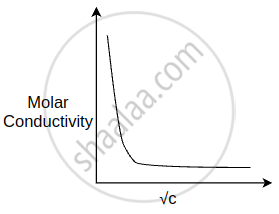
The electrolyte X is ______.
The specific conductance of 2.5 × 10-4 M formic acid is 5.25 × 10-5 ohm-1 cm-1. Calculate its molar conductivity and degree of dissociation.
Given `λ°_("H"^+)` = 349.5 ohm-1 cm2 mol-1 and
`λ°_("HCOO"^-) = 50.5 " ohm"^-1 "cm"^2 "mol"^-1`
