Advertisements
Advertisements
Question
Three electrolytic cells A, B, C containing solutions of \[\ce{ZnSO4}\], \[\ce{AgNO3}\] and \[\ce{CuSO4}\], respectively, are connected in series. A steady current of 1.5 amperes was passed through them until 1.45 g of silver deposited at the cathode of cell B. How long did the current flow? What mass of copper and zinc were deposited?
Solution
\[\ce{Ag^+ + e^- -> Ag}\]
108 g Ag is deposited = 1 F = 96500 C
∴ 1.45 g Ag will be deposited = `96500/108 xx 1.45`
= 1295.6 C
Q = I × t
Or t = `"Q"/"I"`
= `1295.6/1.5`
= 863.7 s
= 14 min 24 s
\[\ce{Cu^{2+} + 2e^- -> Cu}\]
i.e., 2 × 96500 C deposits Cu = 63.5 g
So 1295.6 C will deposit Cu = `(63.5 xx 1295.6)/(2 xx 96500)` = 0.4263 g
Similarly, \[\ce{Zn^{2+} + 2e^- -> Zn}\]
Mass of zinc deposited = `(65.4 xx 1295.6)/(2 xx 96500)`
= 0.44 g
APPEARS IN
RELATED QUESTIONS
The charge of how many coulomb is required to deposit 1.0 g of sodium metal (molar mass 23.0 g mol-1) from sodium ions is -
- 2098
- 96500
- 193000
- 4196
96500 coulombs correspond to the charge on how many electrons?
How much electricity in terms of Faraday is required to produce 20 g of \[\ce{Ca}\] from molten \[\ce{CaCl2}\]?
(Given: Molar mass of Calcium is 40 g mol−1.)
How much charge is required for the following reduction:
1 mol of \[\ce{Al^{3+}}\] to \[\ce{Al}\]?
How much charge is required for the following reduction:
1 mol of \[\ce{MnO^-_4}\] to \[\ce{Mn^{2+}}\]?
State second law of electrolysis
Explain Faraday’s second law of electrolysis
Write the name of the cell which is generally used in transistors. Write the reactions taking place at the anode and the cathode of this cell.
How many faradays of electricity are required to produce 13 gram of aluminium from aluminium chloride solution? (Given: Molar mass of Al = 27.0-gram mol–1)
Following reactions occur at cathode during the electrolysis of aqueous copper(II) chloride solution :
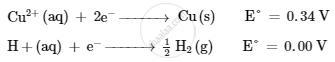
On the basis of their standard reduction electrode potential (E°) values, which reaction is feasible at the cathode and why ?
According to Faraday’s First Law of Electrolysis, the amount of chemical reaction which occurs at any electrode during electrolysis by a current is proportional to the ____________.
In the electrolysis of aqueous sodium chloride solution which of the half cell reaction will occur at anode?
What will happen during the electrolysis of aqueous solution of \[\ce{CuSO4}\] by using platinum electrodes?
(i) Copper will deposit at cathode.
(ii) Copper will deposit at anode.
(iii) Oxygen will be released at anode.
(iv) Copper will dissolve at anode.
Aqueous copper sulphate solution and aqueous silver nitrate solution are electrolysed by 1 ampere current for 10 minutes in separate electrolytic cells. Will the mass of copper and silver deposited on the cathode be same or different? Explain your answer.
Assertion: Electrolysis of NaCl solution gives chlorine at anode instead of O2.
Reason: Formation of oxygen at anode requires overvoltage.
Given `1/a` = 0.5 CM–1, R = 50 ohm, N = 1.0 then equivalent conductance of electrolytic cell is
On passing electricity through nitrobenzene solution, it is converted into azobenzene. The mass of azobenzene is ______ mg, if the same quantity of electricity produces oxygen just sufficient to burn 96 mg of fullerene (C60)·
Assertion (A): During electrolysis of aqueous copper sulphate solution using copper electrodes hydrogen gas is released at the cathode.
Reason (R): The electrode potential of Cu2+/Cu is greater than that of H+/H2.
Select the most appropriate answer from the options given below:
How much electricity in terms of Faraday is required to produce 40.0 g of \[\ce{Al}\] from molten \[\ce{Al2O3}\]?
(Given: Molar mass of Aluminium is 27 g mol−1.)
