Advertisements
Advertisements
Question
Why is Sc3+ colourless while Ti3+ coloured? (Atomic number Sc = 21, Ti =22)
Solution
Ti3+ is a 3d1 system. The colour is due to d–d transition. There is one d electron present in the 3d subshell. When light falls on the Ti3+ complex, the t2g electron is excited to the eg level. This excitation takes place in the greenish yellow region (≈5000 A°) and the rest is transmitted. The complementary colour is transmitted which is violet.
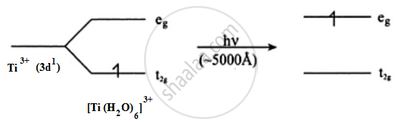
Sc3+ is a 3d0 system. There are no d electrons; hence, d–d transition is not possible. Hence, Sc3+ is colourless.
APPEARS IN
RELATED QUESTIONS
Explain why is Fe3+ more stable than Fe2+?
ln which pair highest oxidation states of transition metals are found:
The elements of 3d transition series are given as: Sc Ti V Cr Mn Fe Co
Answer the following: Write the element which shows maximum number of oxidation states. Give reason.
The elements of 3d transition series are given as: Sc Ti V Cr Mn Fe Co
Answer the following: Which element has the highest m.p?
The elements of 3d transition series are given as: Sc Ti V Cr Mn Fe Co
Answer the following: Which element shows only +3 oxidation state?
Why do the transition elements have higher enthalpies of atomisation?
Account for the following:
E° value for the Mn3+/Mn2+ couple is much more positive than that for Cr3+/Cr2+.
How would you account for the irregular variation of ionization enthalpies (first and second) in the first series of the transition elements?
Why are Mn2+ compounds more stable than Fe2+ towards oxidation to their +3 state?
Calculate the number of unpaired electrons in the following gaseous ions:
Mn3+, Cr3+, V3+ and Ti3+. Which one of these is the most stable in an aqueous solution?
Why do transition metals exhibit higher enthalpy of atomization?
Explain why transition elements form alloys.
Read the passage given below and answer the following question:
The transition metals when exposed to oxygen at low and intermediate temperatures form thin, protective oxide films of up to some thousands of Angstroms in thickness. Transition metal oxides lie between the extremes of ionic and covalent binary compounds formed by elements from the left or right side of the periodic table. They range from metallic to semiconducting and deviate by both large and small degrees from stoichiometry. Since electron bonding levels are involved, the cations exist in various valence states and hence give rise to a large number of oxides. The crystal structures are often classified by considering a cubic or hexagonal close-packed lattice of one set of ions with the other set of ions filling the octahedral or tetrahedral interstices. The actual oxide structures, however, generally show departures from such regular arrays due in part to distortions caused by packing of ions of different size and to ligand field effects. These distortions depend not only on the number of d-electrons but also on the valence and the position of the transition metal in a period or group.
In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage.
Assertion: Crystal structure of oxides of transition metals often show defects.
Reason: Ligand field effect cause distortions in crystal structures.
Highest oxidation state of manganese in fluoride is \[\ce{+4 (MnF4)}\] but highest oxidation state in oxides is \[\ce{+7 (Mn2O7)}\] because ______.
Which of the following will not act as oxidising agents?
(i) \[\ce{CrO3}\]
(ii) \[\ce{MoO3}\]
(iii) \[\ce{WO3}\]
(iv) \[\ce{CrO^{2-}4}\]
Although fluorine is more electronegative than oxygen, but the ability of oxygen to stabilise higher oxidation states exceeds that of fluorine. Why?
Identify A to E and also explain the reactions involved.
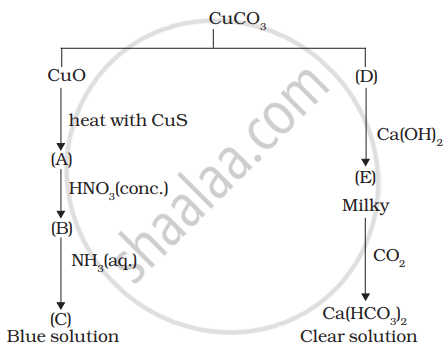
Answer the following question:
Which element of the first transition series has lowest enthalpy of atomisation?
Account for the following:
In case of transition elements, ions of the same charge in a given series show progressive decrease in radius with increasing atomic number.
Why are fluorides of transition metals more stable in their higher oxidation state as compared to the lower oxidation state?
Which one of the following characters tie of the transition metal is associated with higher catalytic activity?
A complex in which dsp2 hybridisation takes place is
Why are all copper halides known except that copper iodide?
Why is the `"E"_(("V"^(3+)//"V"^(2+)))^"o"` value for vanadium comparatively low?
Which one among the following metals of the 3d series has the lowest melting point?
The second ionization enthalpies of chromium and manganese are 1592 and 1509 kJ/mol respectively. Explain the lower value of Mn.
For M2+/M and M3+/M2+systems, the EΘ values for some metals are as follows:
| Cr2+/Cr | −0.9 V |
| Mn2+/Mn | −1.2 V |
| Fe2+/Fe | −0.4 V |
| Cr3/Cr2+ | −0.4 V |
| Mn3+/Mn2+ | +1.5 V |
| Fe3+/Fe2+ | +0.8 V |
Use this data to comment upon:
The ease with which iron can be oxidised as compared to a similar process for either chromium or manganese metal.
