HSC Science (General)
HSC Science (Electronics)
HSC Science (Computer Science)
Academic Year: 2024-2025
Date: March 2025
Advertisements
General Instructions: The question paper is divided into four sections.
- Section A: Q.No.1 contains Ten multiple-choice types of questions carrying One mark each. Q.No.2 contains Eight very short answer type of questions carrying One mark each.
- Section B: Q.No.3 to Q.No.14 are Twelve short answer type questions carrying Two marks each. (Attempt any Eight).
- Section C: Q.No.15 to Q.No.26 are Twelve short answer type questions carrying Three marks each. (Attempt any Eight).
- Section D: Q.No.27 to Q.No.31 are Five Long answer-type questions carrying Four marks each. (Attempt any Three).
- Use of Log table is allowed. Use of calculator is not allowed.
- Figures to the right indicate full marks.
- For each multiple-choice type question, it is mandatory to write the correct answer along with its alphabet. e.g., (a) .............. /(b) ............... /(c) ............... /(d) ................ ,etc. No mark(s) shall be given if ONLY the correct answer or the alphabet of the correct answer is written.
Only the first attempt will be considered for evaluation.
Select the most appropriate option.
A gas is allowed to expand in a well-insulated container against a constant external pressure of 2.5 bar from an initial volume of 2.5 L to a final volume of 4.5 L. The change in internal energy, ΔU of the gas will be _______.
–500 J
+ 500 J
–1013 J
+ 1013 J
Chapter: [0.04] Chemical Thermodynamics
Choose the most correct option.
Formula for the compound sodium hexacynoferrate (III) is ____________.
[NaFe(CN)6]
Na2[Fe(CN)6]
Na[Fe(CN)6]
Na3[Fe(CN)6]
Chapter: [0.09] Coordination Compounds
Polonium has the half-Life of ______.
13.8 days
12 days
5 days
102 days
Chapter: [0.07] Elements of Groups 16, 17 and 18
Choose the most correct option.
The oxidation state of cobalt ion in the complex [Co(NH3)5 Br]SO4 is __________.
+2
+3
+1
+4
Chapter: [0.09] Coordination Compounds
\[\ce{CH2OH-CO-(CHOH)4-CH2OH}\] is an example of ______.
Aldohexose
Aldoheptose
Ketotetrose
Ketoheptose
Chapter: [0.14] Biomolecules
Choose the most correct answer:
Which of the Na following is a buffer solution?
CH3COONa + NaCl in water
CH3COOH + HCl in water
CH3COOH + CH3COONa in water
HCl + NH4Cl in water
Chapter: [0.03] Ionic Equilibria
Which cells is used as a source of power in flashlights?
Dry cell
Fuel cell
Hydrogen cell
None of them
Chapter: [0.05] Electrochemistry
Choose the correct option.
Which of the following is the least acidic compound?
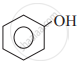
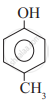
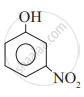
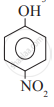
Chapter: [0.11] Alcohols, Phenols and Ethers
The boiling point of HCl is ______.
189 K
200 K
210 K
220 K
Chapter: [7.03] Group 17 Elements
Choose the correct option.
Which of the following substrate will give ionic organic products on reaction?
CH3 – CH2 – OH + Na
CH3 – CH2 – OH + SOCl2
CH3 – CH2 – OH + PCl5
CH3 – CH2 – OH + H2SO4
Chapter: [0.11] Alcohols, Phenols and Ethers
Answer the following in one or two sentences.
Comment on the statement:
no work is involved in an expansion of gas in a vacuum.
Chapter: [0.04] Chemical Thermodynamics
Name one amphoteric solvent.
Chapter: [0.03] Ionic Equilibria
Presence of disulphide link gives rise to which structure of protein?
Chapter: [14.02] Proteins
Answer the following in one sentence.
Name some chain-growth polymers.
Chapter: [0.15] Introduction to Polymer Chemistry
Advertisements
Answer the following in one or two sentences.
What is standard cell potential for the reaction
\[\ce{3Ni_{(s)} + 2Al^{3+} (1M) → 3Ni^{2+} (1M) + 2Al(s)}\], if `E_"Ni"^circ` = –0.25 V and `"E"_("Al")^circ` = –1.66 V?
Chapter: [0.05] Electrochemistry
Answer the following.
Name two gases which deplete ozone layer.
Chapter: [0.07] Elements of Groups 16, 17 and 18
Answer the following.
What is the action of bromine on magnesium metal?
Chapter: [0.07] Elements of Groups 16, 17 and 18
Answer the following in one sentence.
Write reaction showing conversion of benzonitrile into benzoic acid.
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids
Solve
The half-life of a first-order reaction is 1.7 hours. How long will it take for 20% of the reactant to react?
Chapter: [0.06] Chemical Kinetics
Write structural formulae for Pentane-1,4-diol
Chapter: [0.11] Alcohols, Phenols and Ethers [11.01] Alcohols [11.02] Phenols [11.03] Ethers
Write structural formulae for Cyclohex-2-en-1-ol.
Chapter: [0.11] Alcohols, Phenols and Ethers [11.01] Alcohols [11.02] Phenols [11.03] Ethers
Answer the following
Which nanomaterial is used in sunscreen lotion? Write its use.
Chapter: [0.16] Green Chemistry and Nanochemistry
Write two points of difference between the properties of phenol and ethyl alcohol.
Chapter: [0.11] Alcohols, Phenols and Ethers
Give reason:
Reactions involving Grignard reagent must be carried out under anhydrous condition.
Chapter: [0.1] Halogen Derivatives
Write the reaction of conc. H2SO4 with sugar. What is the role of H2SO4 in this reaction?
Chapter: [7.02] Group 16 Elements
Answer the following
What is diazotisation?
Chapter: [0.13] Amines
Answer the following
Write diazotisation reaction of aniline?
Chapter: [0.13] Amines
Answer the following in one or two sentences.
Why all collisions between reactant molecules do not lead to a chemical reaction?
Chapter: [0.06] Chemical Kinetics
Answer the following in one sentence.
Write reaction showing conversion of ethanenitrile into ethanol.
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids [12.01] Aldehydes and Ketones
In NaOH solution [OH–] is 2.87 × 10–4. Calculate the pH of the solution.
Chapter: [0.03] Ionic Equilibria
Give scientific reasons:
On complete hydrolysis DNA gives equimolar quantities of adenine and thymine.
Chapter: [0.14] Biomolecules
Answer the following in brief.
Obtain the relationship between the rate constant and half-life of a first-order reaction.
Chapter: [0.06] Chemical Kinetics
Answer the following question.
Draw geometric isomers and enantiomers of the following complex.
[Pt(en)3]4⊕
Chapter: [0.09] Coordination Compounds
Answer the following question.
Draw geometric isomers and enantiomers of the following complex.
[Pt(en)2ClBr]2⊕
Chapter: [0.09] Coordination Compounds
Draw geometric isomers of the following complex.
Geometrical isomers of Pt(NH3)2Cl2
Chapter: [0.09] Coordination Compounds
Advertisements
Answer the following.
Write structure of natural rubber and neoprene rubber along with the name and structure of their monomers.
Chapter: [0.15] Introduction to Polymer Chemistry [0.15] Polymers
Obtain the relationship between ΔH and ΔU for gas phase reactions.
Chapter: [0.04] Chemical Thermodynamics
Answer the following in brief.
It is impossible to measure the potential of a single electrode. Comment.
Chapter: [0.05] Electrochemistry
Distinguish between SN1 and SN2 mechanism of substitution reaction.
Chapter: [0.1] Halogen Derivatives
Answer the following in brief.
Explain graphically the effect of temperature on the rate of reaction.
Chapter: [0.06] Chemical Kinetics
An element has a bee structure with unit cell edge length of 288 pm. How many unit cells and number of atoms are present in 200 g of the element?
Chapter: [0.01] Solid State
Answer in brief.
What is the action of hydrazine on cyclopentanone in presence of KOH in ethylene glycol?
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids
How ketones are prepared from nitriles?
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids [12.01] Aldehydes and Ketones
Answer the following:
What are anode and cathode for Leclanche' dry cell?
Chapter: [0.05] Electrochemistry
Write electrode reactions and overall cell reaction when Leclanche's dry cell generates electricity.
Chapter: [0.05] Electrochemistry
Answer in brief.
Write reaction showing conversion of Acetaldehyde into acetaldehyde dimethyl acetal.
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids
A solution of citric acid C6H8O7 in 50 g of acetic acid has a boiling point elevation of 1.76 K. If Kb for acetic acid is 3.07 K kg mol-1, what is the molality of solution?
Chapter: [0.02] Solutions
0.01 m aqueous formic acid solution freezes at – 0.021°C. Calculate its degree of dissociation, Kf = 1.86 K kg mol–1.
Chapter: [0.02] Solutions
Answer the following
What are the differences between cast iron, wrought iron, and steel?
Chapter: [0.08] Transition and Inner Transition Elements
Answer the following :
Explain the relation between ionic product and solubility product to predict whether a precipitate will form when two solutions are mixed?
Chapter: [0.03] Ionic Equilibria
Write reaction showing conversion of p-bromoisopropylbenzene into p-isopropylbenzoic acid (3 steps).
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids
Answer the following
Explain trends in ionisation enthalpies of d-block elements.
Chapter: [0.08] Transition and Inner Transition Elements
Answer the following question.
What are cationic, anionic, and neutral complexes? Give one example of each.
Chapter: [0.09] Coordination Compounds
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
Maharashtra State Board previous year question papers 12th Standard Board Exam Chemistry with solutions 2024 - 2025
Previous year Question paper for Maharashtra State Board 12th Standard Board Exam Chemistry-2025 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of Maharashtra State Board 12th Standard Board Exam.
How Maharashtra State Board 12th Standard Board Exam Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
