Advertisements
Advertisements
प्रश्न
A sphere of radius 1.00 cm is placed in the path of a parallel beam of light of large aperture. The intensity of the light is 0.5 W cm−2. If the sphere completely absorbs the radiation falling on it, Show that the force on the sphere due to the light falling on it is the same even if the sphere is not perfectly absorbing.
उत्तर
Consider a sphere of centre O and radius OP. As shown in the figure, the radius OP of the sphere is making an angle θ with OZ. Let us rotate the radius about OZ to get another circle on the sphere. The part of the sphere between the circle is a ring of area `2pir^2sin θdθ`.
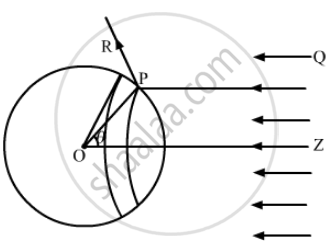
Consider a small part of area `ΔA` of the ring at point P.
Energy of the light falling on this part in time `Δt` ,
`ΔU = I Δ t (ΔA cos θ)`
As the light is reflected by the sphere along PR, the change in momentum ,
`Δp = 2 (ΔU)/c cos θ = 2/c I Δ t (ΔA cos^2 θ)`
Therefore , the force will be
`(Δp)/(Δt) = 2/c I ΔA cos^2 θ`
The Component of force on ΔA , along ZO , is
`(Δp)/(Δt) cos θ = 2/c I ΔA cos^3 θ`
Now , force action on the ring,
`dF = 2/c I (2pir^2 sin θ dθ) cos^3 θ`
The force on the entire sphere ,
`F = ∫_0^(pi/2) (4pir^2I)/c cos^3 θ sin θ dθ`
= `- ∫_0^(pi/2) (4pir^2I)/c cos^3 θd(cos θ)`
= `(pir^2I)/c`
APPEARS IN
संबंधित प्रश्न
Define the term 'intensity of radiation' in terms of photon picture of light.
Ultraviolet light of wavelength 2271 Å from a 100 W mercury source irradiates a photo-cell made of molybdenum metal. If the stopping potential is −1.3 V, estimate the work function of the metal. How would the photo-cell respond to a high intensity (∼105 W m−2) red light of wavelength 6328 Å produced by a He-Ne laser?
Every metal has a definite work function. Why do all photoelectrons not come out with the same energy if incident radiation is monochromatic? Why is there an energy distribution of photoelectrons?
What is the speed of a photon with respect to another photon if (a) the two photons are going in the same direction and (b) they are going in opposite directions?
Can a photon be deflected by an electric field? Or by a magnetic field?
It is found that yellow light does not eject photoelectrons from a metal. Is it advisable to try with orange light or with green light?
Let nr and nb be the number of photons emitted by a red bulb and a blue bulb, respectively, of equal power in a given time.
Light of wavelength λ falls on a metal with work-function hc/λ0. Photoelectric effect will take place only if
An atom absorbs a photon of wavelength 500 nm and emits another photon of wavelength 700 nm. Find the net energy absorbed by the atom in the process.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
Find the maximum kinetic energy of the photoelectrons ejected when light of wavelength 350 nm is incident on a cesium surface. Work function of cesium = 1.9 eV
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
The work function of a photoelectric material is 4.0 eV. (a) What is the threshold wavelength? (b) Find the wavelength of light for which the stopping potential is 2.5 V.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
The electric field associated with a monochromatic beam is 1.2 × 1015 times per second. Find the maximum kinetic energy of the photoelectrons when this light falls on a metal surface whose work function is 2.0 eV.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
The electric field associated with a light wave is given by `E = E_0 sin [(1.57 xx 10^7 "m"^-1)(x - ct)]`. Find the stopping potential when this light is used in an experiment on photoelectric effect with the emitter having work function 1.9 eV.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
A small piece of cesium metal (φ = 1.9 eV) is kept at a distance of 20 cm from a large metal plate with a charge density of 1.0 × 10−9 C m−2 on the surface facing the cesium piece. A monochromatic light of wavelength 400 nm is incident on the cesium piece. Find the minimum and maximum kinetic energy of the photoelectrons reaching the large metal plate. Neglect any change in electric field due to the small piece of cesium present.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
Do all the electrons that absorb a photon come out as photoelectrons?
Consider a thin target (10–2 cm square, 10–3 m thickness) of sodium, which produces a photocurrent of 100 µA when a light of intensity 100W/m2 (λ = 660 nm) falls on it. Find the probability that a photoelectron is produced when a photons strikes a sodium atom. [Take density of Na = 0.97 kg/m3].
Consider a 20 W bulb emitting light of wavelength 5000 Å and shining on a metal surface kept at a distance 2 m. Assume that the metal surface has work function of 2 eV and that each atom on the metal surface can be treated as a circular disk of radius 1.5 Å.
- Estimate no. of photons emitted by the bulb per second. [Assume no other losses]
- Will there be photoelectric emission?
- How much time would be required by the atomic disk to receive energy equal to work function (2 eV)?
- How many photons would atomic disk receive within time duration calculated in (iii) above?
- Can you explain how photoelectric effect was observed instantaneously?
Why it is the frequency and not the intensity of the light source that determines whether the emission of photoelectrons will occur or not? Explain.
What is the effect of threshold frequency and stopping potential on increasing the frequency of the incident beam of light? Justify your answer.
