Advertisements
Advertisements
प्रश्न
Write a condition under which a bimolecular reaction is kinetically first order. Give an example of such a reaction. (Given : log2 = 0.3010,log 3 = 0.4771, log5 = 0.6990).
उत्तर
\[\ce{ C_12 H22O11 + H2O ->[H+] C6 + \underset{Glucose}{C6H12O6} + \underset{Fructose}{C6H12O6} }\] Rate=K[C12H22O4]
Biomolecular reaction is Kinetically first order. Consider a chemical reaction between two substances when one reactanct is present in large excess.
`CH_3 COOC_2H5 +H_2O -> CH_3OOH + C_2H_5OH`
t=0 0.01mol 10mol 0 0
t 0mol 9.9mol 0.01 0.01 mol
Rate = K[CH3COOC2H5]
`Rate = K[CH_3COOC_2H_5] where K=K' [H2O]` the reaction behaves as first order reactions.
APPEARS IN
संबंधित प्रश्न
Explain a graphical method to determine activation energy of a reaction.
Consider a certain reaction \[\ce{A -> Products}\] with k = 2.0 × 10−2 s−1. Calculate the concentration of A remaining after 100 s if the initial concentration of A is 1.0 mol L−1.
Define activation energy.
Calculate activation energy for a reaction of which rate constant becomes four times when temperature changes from 30 °C to 50 °C. (Given R = 8.314 JK−1 mol−1).
The chemical reaction in which reactants require high amount of activation energy are generally ____________.
Which of the following graphs represents exothermic reaction?
(a)
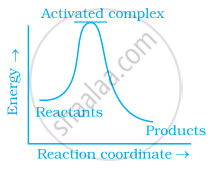
(b)
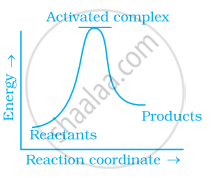
(c)
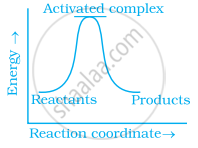
Which of the following statements are in accordance with the Arrhenius equation?
(i) Rate of a reaction increases with increase in temperature.
(ii) Rate of a reaction increases with decrease in activation energy.
(iii) Rate constant decreases exponentially with increase in temperature.
(iv) Rate of reaction decreases with decrease in activation energy.
The reaction between \[\ce{H2(g)}\] and \[\ce{O2(g)}\] is highly feasible yet allowing the gases to stand at room temperature in the same vessel does not lead to the formation of water. Explain.
Why in the redox titration of \[\ce{KMnO4}\] vs oxalic acid, we heat oxalic acid solution before starting the titration?
What happens to the rate constant k and activation energy Ea as the temperature of a chemical reaction is increased? Justify.
