Advertisements
Advertisements
प्रश्न
Solve the following.
A hot air balloon has a volume of 2800 m3 at 99°C. What is the volume if the air cools to 80°C?

उत्तर
Given:
V1 = Initial volume = 2800 m3,
T1 = Initial temperature = 99°C = 99 + 273.15 = 372.15 K,
T2 = Final temperature = 80°C = 80 + 273.15 K = 353.15 K
To find: V2 = Final volume
Formula: `"V"_1/"T"_1="V"_2/"T"_2` (at constant n and P)
Calculation:
According to Charles’ law,
`"V"_1/"T"_1="V"_2/"T"_2` (at constant n and P)
∴ V2 = `("V"_1"T"_2)/"T"_1=(2800xx353.15)/372.15` = 2657 m3
The volume of the balloon when the air cools to 80°C is 2657 m3
APPEARS IN
संबंधित प्रश्न
State the following:
The absolute temperature of a gas at 7°C
Convert the following pressure value into Pascals.
10 atmosphere
Convert the following pressure value into Pascals.
1 atmosphere
Convert 89 kPa to newton per square metre (Nm−2)
Convert 101.325 kPa to bar.
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
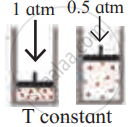 |
______________ |
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
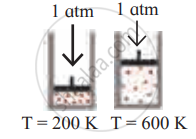 |
______________ |
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
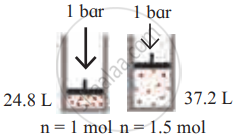 |
______________ |
Consider a sample of a gas in a cylinder with a movable piston.
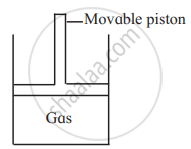
Show diagrammatically the changes in the position of the piston, if the temperature is decreased from 300 K to 150 K at constant pressure.
Consider a sample of a gas in a cylinder with a movable piston.

Show diagrammatically the changes in the position of the piston, if the temperature is decreased from 400 K to 300 K, and pressure is decreased from 4 bar to 3 bar.
Write the statement for Boyle’s law
Solve the following.
A balloon is inflated with helium gas at room temperature of 25°C and at 1 bar pressure when its initial volume is 2.27L and allowed to rise in the air. As it rises in the air external pressure decreases and the volume of the gas increases till finally, it bursts when external pressure is 0.3bar. What is the limit at which the volume of the balloon can stay inflated?
Use of hot air balloon in sports and meteorological observation is an application of
State Boyle's law.
Explain the following observation.
Aerated water bottles are kept under water during summer
Explain the following observation.
The size of a weather balloon becomes larger and larger as it ascends up to larger altitude
A sample of gas has a volume of 8.5 dm3 at an unknown temperature. When the sample is submerged in ice water at 0°C, its volume gets reduced to 6.37 dm3. What is its initial temperature?
A small bubble rises from the bottom of a lake where the temperature and pressure are 6°C and 4 atm. to the water surface, where the temperature is 25°C and pressure is 1 atm. Calculate the final volume in (mL) of the bubble, if its initial volume is 1.5 mL.
According to Andrews isothermals, the minimum temperature at which carbon dioxide gas obeys Boyles law is ______.
Volume of a balloon at 25°C and 1 bar pressure is 2.27 L. If the pressure of the gas in balloon is reduced to 0.227 bar, what is the rise in volume of a gas?
Isochor is the graph plotted between ______.
A gas occupies a volume of 4.2 dm3 at 101 kPa pressure. What volume will gas occupy if the pressure is increased to 235 kPa keeping the temperature constant?
The volume of 400 cm3 chlorine gas at 400 mm of Hg is decreased to 200 cm3 at constant temperature. What is the new pressure of gas?
At what temperature, the volume of gas would become zero?
